Atom Element Compound the basics
advertisement
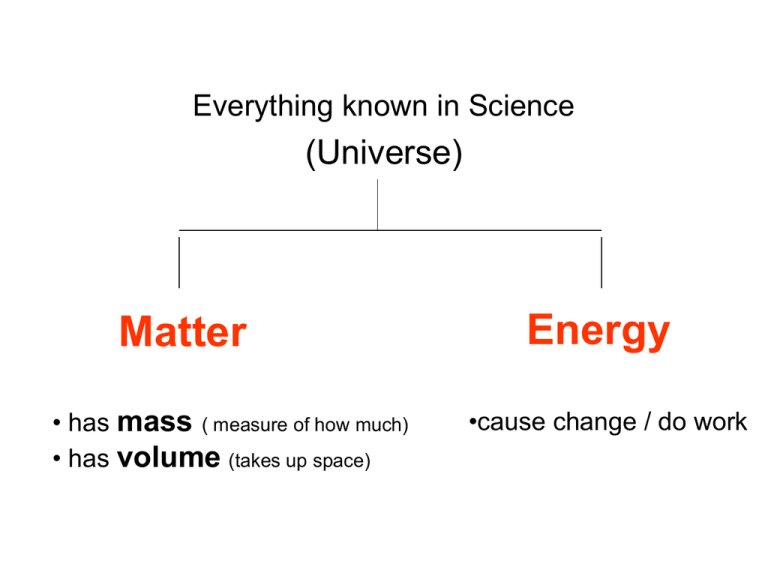
Everything known in Science (Universe) Matter • has mass ( measure of how much) • has volume (takes up space) Energy •cause change / do work Matter - always built from Atoms Atoms – smallest complete units of matter Atoms - made of 3 smaller (subatomic) particles • Protons • Neutrons • Electrons Nucleus = center Helium atom Periodic table of the Elements Different atoms become the foundation for different elements Periodic table of Elements? Elements or Atoms? Element = a pure collection of the exact same type of atoms. Pure = only one type of atom is present Because they are built on the smallest whole unit of matter…the atom… Elements cannot be broken down into different simpler substances !!!! What is the symbol that represents the element Carbon? The capital letter ‘C’ What is the symbol that represents the element Helium? The capital letter ‘H’ followed by a lowercase ‘e’ The symbol for an element will ALWAYS START WITH A CAPITAL letter and… it will have only ONE capital letter…(it may have a lower case letter). Is this a model for an Element? No…it’s got more than one type of atom This is the compound - H2O Compounds = two or more elements that are chemically bonded together Because the different types atoms are chemically bonded… Compounds are NOT MIXTURES! The properties of a compound are different than those of the element from with they are formed. EX: Hydrogen an explosive gas…Oxygen a clear gas that supports combustion. H2O – clear liquid that puts out fire! This model for has lines to help show that the elements H and O are chemically bonded together. Think of these bonds as the energy bonds required to hold the elements together. TWO OR MORE ! H2O H2O NaCl NaCl C12H22O11 Can a compound be pure? Sure…as long as only that compound is present. If it’s all H2O…it’s pure Summary – Element vs. Compound Elements are represented by SYMBOLS. They always Start w/capital & have only one capital. EX: O = Oxygen Fe = Iron Because they are made from one type of atom…Elements cannot be broken down into different simpler substances !!!! Compounds are represented by FORMULAS The formula is a recipe that shows what elements are chemically bonded together in the compound. EX: H2O = 2 Hydrogen bonded with 1 oxygen CO2 = 1 Carbon bonded with 2 oxygen. Because they are made from two or more types of atoms….compound can be broken into simpler substances. Which is an element? Which is a compound? A A = Element One type of atom B B = Compound More than one type of element Which is pure? A B Both ! Are these containers pure? B A NO !








