HDL Cholesterol No Longer Is Good
advertisement

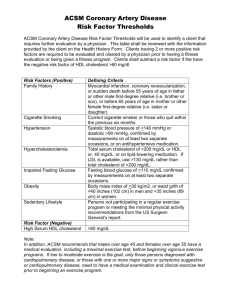
HDL Cholesterol No Longer Is Good Cholesterol: Emerging Genetic Theories Sunita Dodani & Janice S Dorman University of Pittsburgh (Study proposal) Presentation Overview • Study background & significance – Basic description & function of lipids, lipoproteins and apoproteins – HDL & apoprotein A-1 – New theories of LDL & HDL role in atherosclerosis – Concept of dysfunctional HDL – Hypothesized causes of dysfunctionalHDL • Study Rationale • Study objectives • Study Design & Methods Study background & significance • Fats are triacylglycerols containing saturated fatty acids - solid at room temp - usually from animal source (however, coconut & palm oil are saturated). • Oils are triacylglycerols containing monoor polyunsaturated fatty acids - liquid at room temp - usually from plant sources (however, fish oils are polyunsaturated). • Phospholipids are triacylglycerols that have a FA replaced with a phosphate linked FA group. • The major dietary sterol is cholesterol. Functions Of Lipids • Major components of cell membranes. • Required to solubilise fat soluble vitamins • Biosynthetic precursors (e.g. steroid hormones from cholesterol) • Protection (e.g. kidneys are shielded with fat in fed state) • Insulation Lipid transport in the circulation Proteins (apoproteins) Cholesterol HO O R O HO HO R Non polar lipids in core (TAG and cholesterol esters) Lipids are insoluble in plasma. In order to be transported they are combined with specific proteins to form lipoproteins Lipoproteins • Particles found in plasma that transport lipids including cholesterol • Spherical particles with a hydrophobic core (TG and esterified cholesterol) • Apolipoproteins on the surface • large: apoB (b-48 and B-100) atherogenic • smaller: apoA-I, apoC-II, apoE • Classified on the basis of density (NMR spectroscopy) and electrophoretic mobility (VLDL; LDL; IDL;HDL; Lp-a) Five classes of lipoprotein (all contain characteristic amounts TAG, cholesterol, cholesterol esters, phospholipids and Apoproteins – NMR Spectroscopy) Increasing density Class Diameter (nm) Source and function Major apoliproteins Chylomicrons (CM) 500 Intestine. Transport of dietary TAG A, B48, C(I,II,III) E Very low density lipoproteins (VLDL) 43 Liver. Transport of endogenously synthesised TAG B100, C(I,II,III) , E Low density lipoproteins (LDL) 22 Formed in circulation by partial breakdown of IDL. Delivers cholesterol to peripheral tissues B100 High density lipoproteins (HDL) 8 Liver. Removes “used” cholesterol from tissues and takes it to liver. Donates apolipoproteins to CM and VLDL A, C(I,II,III), D, E Lipoprotein class Density (g/mL) Diameter (nm) Protein % of dry wt Phospho lipids % Triacylglycerols % of dry wt HDL 1.0631.21 5 – 15 33 29 8 LDL 1.019 – 1.063 18 – 28 25 21 4 IDL 1.0061.019 25 - 50 18 22 31 VLDL 0.95 – 1.006 30 - 80 10 18 50 Chylomicrons < 0.95 100 - 500 1 - 2 7 84 Composition and properties of human lipoproteins Atherogenic Particles MEASUREMENTS: VLDL VLDLR TG-rich lipoproteins Apolipoprotein B Non-HDL-C IDL LDL Small, dense LDL The Apolipoproteins • Major components of lipoproteins • Often referred to as aproteins • Classified by alphabetical designation (A thru E) • The use of roman numeral suffix describes the order in which the Apolipoproteins emerge from a chromatographic column • Responsible for recognition of particle by receptors Apoproteins of human lipoproteins • A-I (28,300)- principal protein in HDL • 90 –120 mg% in plasma • A-II (8,700) – occurs as dimer mainly in HDL • 30 – 50 mg %; enhances hepatic lipase activity • B-48 (240,000) – found only in chylomicrons – <5 mg %; derived from apo-B-100 gene by RNA editing; lacks the LDL receptorbinding domain of apo-B-100 • B-100 (500,000) – principal protein in LDL • 80 –100 mg %; binds to LDL receptor (Circulation. 2004 Jun 15;109(23 Suppl):III2-7) Apoproteins of human lipoproteins • C-I (7,000) – found in chylomicrons, VLDL, HDL • 4 – 7 mg %; may also activate LCAT • C-II (8,800) - found in chylomicrons, VLDL, HDL • 3 – 8 mg %; activates lipoprotein lipase • C-III (8,800) - found in chylomicrons, VLDL, IDL, HDL • 8 15 mg %; inhibits lipoprotein lipase • D (32,500) - found in HDL • 8 – 10 mg %; also called cholesterol ester transfer protein (CETP) • E (34,100) - found in chylomicrons, VLDL, IDL HDL • 3 – 6 mg %; binds to LDL receptor • H (50,000) – found in chylomicrons; also known as b-2-glycoprotein I (involved in TG metabolism) Major lipoprotein classes Chylomicrons • Formed through extrusion of resynthesized triglycerides from the mucosal cells into the intestinal lacteals • Flow through the thoracic ducts into the subclavian veins • Degraded to remnants by the action of lipoprotein lipase (LpL) which is located on capillary endothelial cell surface • Remnants are taken up by liver parenchymal cells Major lipoprotein classes • VLDL – – – – – – density >1.006 diameter 30 - 80nm endogenous triglycerides apoB-100, apoE, apoC-II/C-III prebeta in electrophoresis formed in the liver as nascent VLDL (contains only triglycerides, apoE and apoB) This animation shows how VLDL are metabolised once they enter the circulation from the liver Tissues B100 VLDL B100 Lipoprotein LDL lipase E CII Some LDL taken up by liver (LDL receptors) Having lost TAG to tissues LDL contains a large proportion of cholesterol/cholesterol esters Capillary wall (endothelial surface) Some LDL taken up by other tissues (LDL receptors). LDL delivers cholesterol and TAG to the extra hepatic tissues. LDL membrane receptor • Found in clathrin coated pits (endocytosis) • After endocytosis the receptor is recycled whilst the LDL is degraded to releasing lipid cargo. Cholesterol uptake down regulates the cells own production of cholesterol and down regulates LDL receptor synthesis • Mutations in LDL receptors causes increased plasma LDL levels (i.e. increased cholesterol levels). This accelerates progress of atherosclerosis (Familial hyperlipedimias). • The cholesterol in LDL is often called “bad cholesterol”. LDL Heterogeneity: Small vs. large LDL Production of Small Dense LDL Although size (& lipid content) changes, there is always 1 molecule apo B protein / particle Lipoprotein receptors B B B B Lipase TG CE VLDL Oxidation & modification (CETP) VLDL LipidsIDL largeLDL dense remnants transferred 1 2 3 Relative Atherogenicity of Large and Small LDL Particles B B CE CE Tg Tg High density lipoprotiens • Act as a reservoir for apoproteins which can be donated or received from other lipoproteins. • Also play a vital role in scavenging “used” cholesterol (reverse cholesterol transport): apoproteins HDL HDL HDL receptor mediated endocytosis by liver HDL Liver “used” cholesterol transferred to HDL and converted to cholesterol ester some cholesterol ester transferred to circulating VLDL VLDL LDLreceptor Peripheral mediated LDL LDL tissues endocytosis Cholesterol can be converted to bile salts for excretion or repackaged in VLDL for redistribution High density lipoprotiens HDL • HDL carries “used” cholesterol (as CE) back to the liver. Also donate some CE to circulating VLDL for redistribution to tissues. • HDL taken up by liver and degraded. The cholesterol is excreted as bile salts or repackaged in VLDL for distribution to tissues. • Cholesterol synthesis in the liver is regulated by the cholesterol arriving through HDL (and dietary cholesterol returned by chylomicrons remnants). • Cholesterol (CE) in HDL is referred to as “good cholesterol” Helical Wheel Projection Of A Portion Of Apolipoprotein A-1 HDL functioning • HDL may transfer some cholesterol esters to other lipoproteins. • Some remain associated with HDL, which may be taken up by liver & degraded. • HDL thus transports cholesterol from tissues & other lipoproteins to the liver, which can excrete excess cholesterol as bile acids. • High blood levels of HDL (the "good" cholesterol) correlate with low incidence of atherosclerosis HDL > 40 mg/dl (NCEP ATP III) “Independent Predictor of CAD” Interrelationship between lipoproteins VLDL FFA CE CETP LPL Liver (LDL receptor) TG IDL TG CETP CE LPL HDL TG FFA CETP Liver (LDL receptor) CE LDL Reverse Cholesterol Transport: Indirect Extra hepatic tissues Liver Cholesterol is reused or excreted in bile Cholesterol esters hydrolysis Direct Free cholesterol ABCA1 Pre-b-HDL A LCAT A HDL CETP Cholesterol to VLDL, IDL,LDL Postprandial Changes in Plasma Lipid Metabolism Fat storage via LPL Transfer of cholesterol from cells into plasma reverse transport of cholesterol from peripheral tissue to liver Exchange of cholesterol for VLDL TG in HDL (CETP) LCAT activity = esterification of free cholesterol (HDL) These postprandial changes are beneficial in maintaining whole body homeostasis of glycerides and cholesterol 1. LCAT deficiency? 2. CETP deficiency? 3. Apo AI deficiency? LDL Liver Dietary fat Bile salts small intestine chylomicrons Endogenous cholesterol extra hepatic tissue Exogenous cholesterol chylomicrons reminants HDL VLDL IDL capillaries Lipoprotein Lipase Lipoprotein Lipase FFA Adipose, muscle FFA ATHEROSCLEROSIS Normal Intima Atherosclerosis Proliferation of intimal connective tissue Medis Adventitis Lipid accumulation Tissue breakdown Factors such as high plasma cholesterol, smoking, hypertension, diabetes and family history are all associated with atherosclerosis. Progression Of Atherosclerosis Role of LDL in atherosclerosis- oxidation Monocycle Cholesterol ester LDL Endothelial injury Platelets Foam cells N-LDL B OX-LDL B Oxidized LDL LUMEN Endothelium LDL, VLDL Free radicals INTIMA ? IEL Concentration MEDIA Residence time in arterial wall Opportunity to be oxidized, taken up by macrophage, glycated and be trapped Modified protein & lipid = immunogenicity Oxidized LDL B B CE CE Tg CE CE CE Tg CE LDL C Apo B B CE CE Tg CE 3.5 75 B B CE CE Tg CE CE CE Tg CE B B CE CE CE CE Tg CE Tg CE B B CE CE CE CE Tg CE Tg CE 3.5 150 LDL apoB Percent 40 30 20 10 0 40 25 small dense LDL is more toxic ( oxidation etc.) but has less cholesterol per particle, measuring mg/dl LDL cholesterol doesn’t give complete picture. 60 80 100 120 140 160 180 200 220 240 260 280 LDL cholesterol 20 15 10 5 0 mmol/l 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 Controls CHD patients Measuring apoB provides a better index of particle number, and an additional discriminator. Macrophage induced inflammation Rupture 7 Endothelium Fatty streak Monocyte LDL Adhesion 1 X-LAM 2 LDL Lipid Oxidation MM-LDL Oxidation Ox-LDL MCP-1 Entry 3 M-CSF MCP-1 M-CSF IL-1 5 Differentiation Modified LDL uptake ROS 4 Macrophage Smooth Muscle cells Smooth muscle cell proliferation 6 Development of AtherosclerosisLumen Intima (Endothelial damage) Fatty lesion Risk factors Regression fibro lipid plaque formation Fibrous cap •Ground substance •Collagen fibers •Reticulum fibrils Thrombosis Ulceration 1 2 3 Progressive Reduction in blood flow R Smooth Foam Calcification Muscle cells Intercellular lipid Lipid core cells Haemorrhage Fatty streak Fibrous plaque Complicated lesion Lumen Diameter 100% High LDL Levels Non-Specific Receptor Mediated Pathway Anti-oxidant therapy not effective Relationship between HDL/LDL and heart disease Monocyte (white blood cell) Cholesterol to liver LDL vascular endothelium (+) differentiate Oxidized LDL Macrophage LDL (+) (-) HDL Foam cells (fatty streak) Arterial Intima Role played by Apo A1 HDL Function • • • • Removal of CE from LDL Reverse Cholesterol transport Apo A-1 prevent seeding of LDL Apo A-1 prevent oxidized LDL formation HDL NO More a Good Cholesterol Recent theories Framingham study of the incidence of coronary heart disease (CHD) & HDL:44% of the events occurred in men with HDLcholesterol levels of 40 mg/dl and 43% of the events occurred in women with HDLcholesterol levels of 50 mg/dl A significant number of CHD events occur in patients with normal LDL-cholesterol levels and normal HDL-cholesterol levels. Search for markers with better predictive value HDL NO More Good Cholesterol Increase CHD on high HDL Good HDL becomes bad (Navab M, 2002) Conversion of anti-inflammatory HDL into pro-inflammatory HDL Increase risk of atherosclerosis Dysfunctional HDL has been detected by special test of cell-free assay (Navab M, 2002) Non-functioning Apo A1 “What makes HDL dysfunctional”? HDL NO More Good Cholesterol Recent hypotheses 1. Products of an inflammatory enzyme, myeloperoxidase target main Apo A1 Converting HDL into pro-inflammatory non-functional. 2. Apo A1 macrophages retain increase cholesterol & cholesterol reverse transport reduced. Myeperoxidase modify tyrosine AA (Fogelman AM 2003) 3. Dysfunctional HDL has increases hydroperoxidase This makes it proinflammatory (Van Lenten et al J. Clin. Invest. 96: 2758–2767 ) HDL NO More Good Cholesterol Role of myeloperoxidase: Modify tyrosine AA in Apo A-1 100 X more than same AA in other protein Study: In patients with CHD there is substantial amount of tyrosine AA in Apo A1 modified by myeloperoxidase than in controls (Zheng et al 2004) Why this occur ??? Study Rationale Un answered Questions 1. Which patients are susceptible to develop dysfunctional HDL 2. What makes myeloperoxidase to cause change in Apo A1 3. What is the role of hydroperoxidase in causing dysfunctional HDL Study Rationale Un answered Questions 1. Which patients are susceptible to develop dysfunctional HDL 2. What makes myeloperoxidase to cause change in Apo A1 3. What is the role of hydroperoxidase in causing dysfunctional HDL Study Proposal Objectives are to: 1. Measure the level of functional & dysfunctional HDL in CHD cases and controls 2. Assess the risk factors association with dysfunctional HDL in both cases and controls 3. Measure the levels of myeloperoxidase, hydroperoxidase and Apo A-1 protein in both cases and controls 4. Study the candidate genes for myeloperoxidase, hydroperoxidase and Apo A-1in cases and controls Study Proposal Study Design: Case-control Study population: South Asian Immigrant population residing in San Diego, California (total population1500 families) Number of Groups 1. Hindus: from India, Nepal & SriLanka Ethnic groups: Gujarati, Marathi, Hindi 2. Muslims: from Pakistan, India & Bangladesh Study Proposal Cases: with known CAD Controls: without CAD Risk factors under study 1. Traditional risk factors 2. Emerging risk factors 3. Myeloperoxidase levels 4. Hydroperoxidase levels 5. Apo A-1 levels Study Proposal Sample size: Biostatistician Data analysis: Multiple logistic regression