07. Metabolism of lipids 1
advertisement

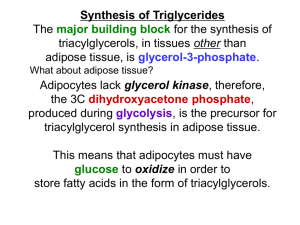
METABOLISM OF LIPIDS: DIGESTION OF LIPIDS. TRANSPORT FORMS OF LIPIDS PHYSIOLOGICAL ROLE OF LIPIDS Energetic role (fuel molecules) Components of membranes (structural role) Precursors for many hormones (steroids) Signal molecules (prostaglandins) Protective role (lipids surround important organs) Enzyme cofactors (vitamin K) Electron carriers (ubiquinone) Insulation against temperature extremes TRIACYLGLYCEROLS ARE HIGHLY CONCENTRATED ENERGY STORES •Triacylglycerols (TGs) and glycogen two major forms of stored energy TGs which are more efficient energy stores because: (1) They are stored in an anhydrous form (2) Their fatty acids are more reduced than monosaccharides. • 1 g of triacylglycerols stores more than six times as much energy as a 1 g of glycogen • Glycogen reserves are depleted in 12 to 24 hours after eating, triacylglycerols within several weeks. •Fat breakdown about 50 % of energy in liver, kidney and skeletal muscles up to 95 % of energy cardiac muscle •Fats are the major source of energy for: fasting animal organism in diabetes • Fatty acids and glycerol substances that are directly used as a fuel by mammalian organisms. • Fatty acids (FA) and glycerol for metabolic fuels are obtained from triacylglycerols: (1) In the diet (2) Stored in adipocytes (fat storage cells) • Free fatty acids occur only in trace amounts in cells •For supplying of fatty acids as a fuel for organism, the triacylglycerols have to be digested DIGESTION OF DIETARY LIPIDS Lipids in diet: triacylglycerols phospholipids cholesterol Digestion – in small intestine. Enzyme – pancreatic lipase. Lipase catalyzes hydrolysis at the C1 and C3 positions of TGs producing free fatty acids and 2-monoacylglycerol. Colipase – protein which is present in the intestine and helps bind the water-soluble lipase to the lipid substrates. Colipase also activates lipase. Bile salts (salts of bile acids) are required for lipids digestion. Bile salts are synthesized in the liver from cholesterol. Taurocholate and glycocholate - the most abundant bile salts. Amphipathic: hydrophilic (blue) and hydrophobic (black) TGs are water insoluble and lipase is water soluble. Digestion of TGs takes place at lipid-water interfaces. Rate of digestion depends on the surface area of the interface. Bile salts are amphipathic, they act as detergent emulsifying the lipid drops and increasing the surface area of the interface. Bile salts also activates the lipase. Inadequate production of bile salts results in steatorrhea. Dietary phospholipids are degraded by phospholipases Phospholipases are synthesized in the pancreas. Major phospholipase is phospholipase A2 (catalyses the hydrolysis of ester bond at C2 of glycerophospholipids and lysophosphoglycerides are formed). Lysophosphoglycerides are absorbed and in the intestinal cells are reesterified back to glycerophospholipids. Lysophosphoglycerides can act as detergent and therefore in high concentration can disrupt cellular membranes. Lysophosphoglyceride is normally present in cells in low concentration. Snake venom contain phospholipase A2 and causes the lysis of erythrocytes membranes. Dietary cholesterol • Most dietary cholesterol is unesterified • Cholesteryl esters are hydrolyzed in the intestine by an intestinal esterase • Free cholesterol is solublized by bile-salt micelles for absorption • After absorption in the intestinal cells cholesterol react with acyl-CoA to form cholesteryl ester. ABSORPTION OF DIETARY LIPIDS Lipid absorption – passive diffusion process. 2-monoacylglycerols, fatty acids, lysophosphoglycerides, free cholesterol form micelles with bile salts. Micelles migrate to the microvilli and lipids diffuse into the cells. Bile acids are actively absorbed and transferred to the liver via portal vein. Bile salts can circulate through intestine and liver several time per day. In the intestinal cells the fatty acids are converted to fatty acyl CoA molecules. Three of these molecules can combine with glycerol, or two with monoacylglycerol, to form a triacylglycerols. O 1. CH2 OH CH O CH2 OH O C R2 + R1 CO SCoA CH2 O C O CH O C CH2 OH 2. O C O CH O C CH2 OH R2 + HSCoA O O CH2 R1 R1 R2 + R3 CO SCoA CH2 O C O R1 CH O C O R2 + HSCoA CH2 O C R3 1-st reaction is catalyzed by monoacylglycerol acyltransferase 2-nd reaction is catalyzed by diacylglycerol acyltransferase TRANSPORT FORMS OF LIPIDS • TGs, cholesterol and cholesterol esters are insoluble in water and cannot be transported in blood or lymph as free molecules • These lipids assemble with phospholipids and apoproteins (apolipoproteins) to form spherical particles called lipoprotein Structure: Hydrophobic core: -TGs, -cholesteryl esters Hydrophilic surfaces: -cholesterol, -phospholipids, -apolipoproteins The main classes of lipoproteins 1.Chylomicrons. 2.Very low density lipoproteins (VLDL). 3.Intermediate density lipoproteins (IDL). 4.Low density lipoproteins (LDL). 5.High density lipoproteins (HDL). Chylomicrons • are the largest lipoproteins (180 to 500 nm in diameter) • are synthesized in the ER of intestinal cells • contain 85 % of TGs (it is the main transport form of dietary TGs). • apoprotein B-48 (apo B-48) is the main protein component • deliver TGs from the intestine (via lymph and blood) to tissues (muscle for energy, adipose for storage). • bind to membrane-bound lipoprotein lipase (at adipose tissue and muscle), where the triacylglycerols are again degraded into free fatty acids and monoacylglycerol for transport into the tissue • are present in blood only after feeding exocytosis Lymphatic vessel • are formed in the liver VLDL • contain 50 % of TGs and 22 % of cholesterol • two lipoproteins — apo B-100 and apo E • the main transport form of TGs synthesized in the organism (liver) • deliver the TGs from liver to peripheral tissue (muscle for energy, adipose for storage) • bind to membrane-bound lipoprotein lipases (triacylglycerols are again degraded into free fatty acids and monoacylglycerol) triacylglycerol cholesteryl esters Apo B Apo E cholesterol phospholipids Lipoproteinlipase – enzyme which is located within capillaries of muscles and adipose tissue Function: hydrolyses of TGs of chylomicrons and VLDL. Formed free fatty acids and glycerol pass into the cells Chylomicrons and VLDL which gave up TGs are called remnants of chylomicrons and remnants of VLDL Remnants are rich in cholesterol esters Remnants of chylomicrons are captured by liver Remnants of VLDL are also called intermediate density lipoproteins (IDL) Fate of the IDL: - some are taken by the liver - others are degraded to the low density lipoproteins (LDL) (by the removal of more triacylglycerol) LDL LDL are formed in the blood from IDL and in liver from IDL (enzyme – liver lipase) LDL are enriched in cholesterol and cholesteryl esters (contain about 50 % of cholesterol) Protein component - apo B-100 LDL is the major carrier of cholesterol (transport cholesterol to peripheral tissue) Cells of all organs have LDL receptors Receptors for LDL are localized in specialized regions called coated pits, which contain a specialized protein called clathrin Apo B-100 on the surface of an LDL binds to the receptor Receptor-LDL complex enters the cell by endocytosis. Endocytic vesicle is formed Vesicle fuse with lysosomes Lysosomal lipases and proteases degrade LDL LDL receptor itself returns to the plasma membrane Apo B-100 is hydrolyzed to amino acids Cholesteryl esters are hydrolyzed to free cholesterol and fatty acids Released free cholesterol: - is incorporated into the membranes or - is reesterified for storage inside the cell by the enzyme acyl CoA:cholesterol acyltransferase (ACAT) Feedback regulation: abundance of intracellular cholesterol suppresses the synthesis of LDL receptors and so the uptake of additional cholesterol from plasma LDL is blocked LDL uptake by receptor-mediated endocytosis Familial hypercholesterolemia congenital disease when LDL receptor are not synthesized (mutation at a single autosomal locus) the concentration of cholesterol in blood markedly increases severe atherosclerosis is developed (deposition of cholesterol in arteries) nodules of cholesterol called xanthomas are prominent in skin and tendons most homozygotes die of coronary artery disease in childhood the disease in heterozygotes (1 in 500 people) has a milder and more variable clinical course atherosclerosis xanthomas HDL are formed in the liver and partially in small intestine contain the great amount of proteins (about 40 %) pick up the cholesterol from peripheral tissue, chylomicrons and VLDL enzyme acyltransferase in HDL esterifies cholesterols, convert it to cholesterol esters and transport to the liver High serum levels of cholesterol cause disease and death by contributing to development of atherosclerosis Cholesterol which is present in the form of the LDL is so-called "bad cholesterol." Cholesterol in the form of HDL is referred to as "good cholesterol” HDL functions as a shuttle that moves cholesterol throughout the body LDL/HDL Ratio The ratio of cholesterol in the form of LDL to that in the form of HDL can be used to evaluate susceptibility to the development of atherosclerosis For a healthy person, the LDL/HDL ratio is 3.5 Transport Forms of Lipids LIPID METABOLISM: MOBILIZATION OF TRIACYLGLYCEROLS; OXIDATION OF GLYCEROL Storage and Mobilization of Fatty Acids (FA) • TGs are delivered to adipose tissue in the form of chylomicrones and VLDL, hydrolyzed by lipoprotein lipase into fatty acids and glycerol, which are taken up by adipocytes. • Then fatty acids are reesterified to TGs. • TGs are stored in adipocytes. • To supply energy demands fatty acids and glycerol are released – mobilisation of TGs. adipocyte At low carbohydrate and insulin concentrations (during fasting), TG hydrolysis is stimulated by epinephrine, norepinephrine, glucagon, and adrenocorticotropic hormone. TG hydrolysis is inhibited by insulin in fed state •Lipolysis - hydrolysis of triacylglycerols by lipases. •A hormone-sensitive lipase converts TGs to free fatty acids and monoacylglycerol •Monoacylglycerol is hydrolyzed to fatty acid and glycerol or by a hormone-sensitive lipase or by more specific and more active monoacylglycerol lipase Transport of Fatty Acids and Glycerol • Fatty acids and glycerol diffuse through the adipocyte membrane and enter bloodstream. • Glycerol is transported via the blood in free state and oxidized or converted to glucose in liver. • Fatty acids are traveled bound to albumin. • In heart, skeletal muscles and liver they are oxidized with energy release. Oxidation of Glycerol Glycerol is absorbed by the liver. Steps: phosphorylation, oxidation and isomerisation. Glyceraldehyde 3-phosphate is an intermediate in: glycolytic pathway gluconeogenic pathways Isomerase ATP Generation from Glycerol Oxidation glycerol – glycerol 3-phosphate - 1 ATP glycerol 3-phosphate - dihydroxyaceton phosphate 2.5ATP (1 NADH) glyceraldehyde 3-phosphate – pyruvate 4,5 ATP (1NADH + 2 ATP) pyruvate – acetyl CoA 2.5 ATP (1 NADH) acetyl CoA in Krebs cycle 10 ATP (3NADH + 1 FADH2 + 1GTP) Total 19,5-1 = 18,5 ATP LIPID METABOLISM: FATTY ACID OXIDATION Stages of fatty acid oxidation (1) Activation of fatty acids takes place on the outer mitochondrial membrane (2) Transport into the mitochondria (3) Degradation to two-carbon fragments (as acetyl CoA) in the mitochondrial matrix (b-oxidation pathway) (1) Activation of Fatty Acids • Fatty acids are converted to CoA thioesters by acyl-CoA synthetase (ATP dependent) • The PPi released is hydrolyzed by a pyrophosphatase to 2 Pi • Two phosphoanhydride bonds (two ATP equivalents) are consumed to activate one fatty acid to a thioester (2) Transport of Fatty Acyl CoA into Mitochondria • The carnitine shuttle system. • Fatty acyl CoA is first converted to acylcarnitine (enzyme carnitine acyltransferase I (bound to the outer mitochondrial membrane). • Acylcarnitine enters the mitochondria by a translocase. • The acyl group is transferred back to CoA (enzyme carnitine acyltransferase II). • Carnitine shuttle system • Path of acyl group in red (3) The Reactions of b oxidation • The b-oxidation pathway (b-carbon atom (C3) is oxidized) degrades fatty acids two carbons at a time b 1. Oxidation of acyl CoA by an acyl CoA dehydrogenase to give an enoyl CoA Coenzyme - FAD 2. Hydration of the double bond between C-2 and C-3 by enoyl CoA hydratase with the 3-hydroxyacyl CoA (b-hydroxyacyl CoA) formation 3. Oxidation of 3-hydroxyacyl CoA to 3-ketoacyl CoA by 3-hydroxyacyl CoA dehydrogenase Coenzyme – NAD+ 4. Cleavage of 3-ketoacyl CoA by the thiol group of a second molecule of CoA with the formation of acetyl CoA and an acyl CoA shortened by two carbon atoms. Enzyme b-ketothiolase. The shortened acyl CoA then undergoes another cycle of oxidation The number of cycles: n/2-1, where n – the number of carbon atoms b-Oxidation of Fatty acyl CoA saturated fatty acids • One round of b oxidation: 4 enzyme steps produce acetyl CoA from fatty acyl CoA • Each round generates one molecule each of: FADH2 NADH Acetyl CoA Fatty acyl CoA (2 carbons shorter each round) Fates of the products of b-oxidation: - NADH and FADH2 - are used in ETC - acetyl CoA - enters the citric acid cycle - acyl CoA – undergoes the next cycle of oxidation ATP Generation from Fatty Acid Oxidation Net yield of ATP per one oxidized palmitate Palmitate (C15H31COOH) - 7 cycles – n/2-1 • The balanced equation for oxidizing one palmitoyl CoA by seven cycles of b oxidation Palmitoyl CoA + 7 HS-CoA + 7 FAD+ + 7 NAD+ + 7 H2O 8 Acetyl CoA + 7FADH2 + 7 NADH + 7 H+ ATP generated 8 acetyl CoA 7 FADH2 7 NADH 10x8=80 7x1.5=10.5 7x2.5=17.5 108 ATP ATP expended to activate palmitate Net yield: -2 106 ATP LIPID METABOLISM: FATTY ACID OXIDATION b-OXIDATION OF ODD-CHAIN FATTY ACIDS • Odd-chain fatty acids occur in bacteria and microorganisms • Final cleavage product is propionyl CoA rather than acetyl CoA • Three enzymes convert propionyl CoA to succinyl CoA (citric acid cycle intermediate) Propionyl CoA Is Converted into Succinyl CoA 1. Propionyl CoA is carboxylated to yield the D isomer of methylmalonyl CoA. The hydrolysis of an ATP is required. Enzyme: propionyl CoA carboxylase Coenzyme: biotin 2. The D isomer of methylmalonyl CoA is racemized to the L isomer Enzyme: methylmalonyl-CoA racemase 3. L isomer of methylmalonyl CoA is converted into succinyl CoA by an intramolecular rearrangement Enzyme: methylmalonyl CoA mutase Coenzyme: vitamin B12 (cobalamin) OXIDATION OF FATTY ACIDS IN PEROXISOMES Peroxisomes - organelles containing enzyme catalase, which catalyzes the dismutation of hydrogen peroxide into water and molecular oxygen Acyl CoA dehydrogenase transfers electrons to O2 to yield H2O2 instead of capturing the highenergy electrons by ETC, as occurs in mitochondrial boxidation. METABOLISM OF LIPIDS: SYNTHESIS OF FATTY ACIDS Fatty Acid Synthesis • Occurs mainly in liver and adipocytes, in mammary glands during lactation • Occurs in cytoplasm • FA synthesis and degradation occur by two completely separate pathways • When glucose is plentiful, large amounts of acetyl CoA are produced by glycolysis and can be used for fatty acid synthesis Three stages of fatty acid synthesis: A. Transport of acetyl CoA into cytosol B. Carboxylation of acetyl CoA C. Assembly of fatty acid chain A. Transport of Acetyl CoA to the Cytosol • Acetyl CoA from catabolism of carbohydrates and amino acids is exported from mitochondria via the citrate transport system • Cytosolic NADH also converted to NADPH • Two molecules of ATP are expended for each round of this cyclic pathway Citrate transport system Sources of NADPH for Fatty Acid Synthesis 1. One molecule of NADPH is generated for each molecule of acetyl CoA that is transferred from mitochondria to the cytosol (malic enzyme). 2. NADPH molecules come from the pentose phosphate pathway. B. Carboxylation of Acetyl CoA Enzyme: acetyl CoA carboxylase Prosthetic group - biotin A carboxybiotin intermediate is formed. ATP is hydrolyzed. The CO2 group in carboxybiotin is transferred to acetyl CoA to form malonyl CoA. Acetyl CoA carboxylase is the regulatory enzyme. C. The Reactions of Fatty Acid Synthesis • Five separate stages: (1) Loading of precursors via thioester derivatives (2) Condensation of the precursors (3) Reduction (4) Dehydration (5) Reduction During the fatty acid synthesis all intermediates are linked to the protein called acyl carrier protein (ACP-SH), which is the component of fatty acyl synthase complex. The pantothenic acid is a component of ACP. Intermediates in the biosynthetic pathway are attached to the sulfhydryl terminus of phosphopantotheine group. The elongation phase of fatty acid synthesis starts with the formation of acetyl ACP and malonyl ACP. Acetyl transacylase and malonyl transacylase catalyze these reactions. Acetyl CoA + ACP acetyl ACP + CoA Malonyl CoA + ACP malonyl ACP + CoA Condensation reaction. Acetyl ACP and malonyl ACP react to form acetoacetyl ACP. Enzyme acyl-malonyl ACP condensing enzyme. Reduction. Acetoacetyl ACP is reduced to D-3hydroxybutyryl ACP. NADPH is the reducing agent Enzyme: b-ketoacyl ACP reductase Dehydration. D-3-hydroxybutyryl ACP is dehydrated to form crotonyl ACP (trans-2-enoyl ACP). Enzyme: 3-hydroxyacyl ACP dehydratase Reduction. The final step in the cycle reduces crotonyl ACP to butyryl ACP. NADPH is reductant. Enzyme - enoyl ACP reductase. This is the end of first elongation cycle (first round). In the second round butyryl ACP condenses with malonyl ACP to form a C6-b-ketoacyl ACP. Reduction, dehydration, and a second reduction convert the C6-bketoacyl ACP into a C6acyl ACP, which is ready for a third round of elongation. Final reaction of FA synthesis • Rounds of synthesis continue until a C16 palmitoyl group is formed • Palmitoyl-ACP is hydrolyzed by a thioesterase Overall reaction of palmitate synthesis from acetyl CoA and malonyl CoA Acetyl CoA + 7 Malonyl CoA + 14 NADPH + 14 H+ Palmitate + 7 CO2 + 14 NADP+ + 8 HS-CoA + 6 H2O Organization of Multifunctional Enzyme Complex in Eukaryotes The synthase is dimer with antiparallel subunits. Each subunit has three domains. ACP is located in domain 2. Domain 1 contains transacylases, ketoacyl-ACP synthase (condensing enzyme) Domain 2 contains acyl carrier protein, b-ketoacyl reductase, dehydratase, and enoyl reductase. Domain 3 contains thioesterase activity. Fatty Acid Elongation and Desaturation The common product of fatty acid synthesis is palmitate (16:0). Cells contain longer fatty acids and unsaturated fatty acids they are synthesized in the endoplasmic reticulum. The reactions of elongation are similar to the ones seen with fatty acid synthase (new carbons are added in the form of malonyl CoA). For the formation of unsaturated fatty acids there are various desaturases catalizing the formation of double bonds. THE CONTROL OF FATTY ACID METABOLISM Acetyl CoA carboxylase plays an essential role in regulating fatty acid synthesis and degradation. The carboxylase is controlled by hormones: glucagon, epinephrine, and insulin. Another regulatory factors: citrate, palmitoyl CoA, and AMP Global Regulation is carried out by means of reversible phosphorylation Acetyl CoA carboxylase is switched off by phosphorylation and activated by dephosphorylation Insulin stimulates fatty acid synthesis causing dephosphorylation of carboxylase. Glucagon and epinephrine have the reverse effect (keep the carboxylase in the inactive phosphorylated state). Protein kinase is activated by AMP and inhibited by ATP. Carboxylase is inactivated when the energy charge is low. Local Regulation Acetyl CoA carboxylase is allosterically stimulated by citrate. The level of citrate is high when both acetyl CoA and ATP are abundant (isocitrate dehydrogenase is inhibited by ATP). Palmitoyl CoA inhibits carboxylase. Fed state: Response to Diet • Insulin level is increased • Inhibits hydrolysis of stored TGs • Stimulates formation of malonyl CoA, which inhibits carnitine acyltransferase I • FA remain in cytosol (FA oxidation enzymes are in the mitochondria) Starvation: • Epinephrine and glucagon are produced and stimulate adipose cell lipase and the level of free fatty acids rises • Inactivate carboxylase, so decrease formation of malonyl CoA (lead to increased transport of FA into mitochondria and activate the b-oxidation pathway) LIPID METABOLISM: BIOSYNTHESIS OF TRIACYLGLYCEROLS AND PHOSPHOLIPIDS Synthesis of Triacylglycerols (TGs) and Glycerophospholipids (GPLs) Glycerol 3-phosphate can be obtained either by the reduction of dihydroxyecetone phosphate (primarily) or by the phosphorylation of glycerol (to a lesser extent). Formation of phosphatidate Two separate acyl transferases (AT) catalyze the acylation of glycerol 3-phosphate. The first AT (esterification at C1) has preference for saturated fatty acids; the second AT (esterification at C2) prefers unsaturated fatty acids. • Phosphatidic acid (phosphatidate) is an common intermediate in the synthesis of TGs and GPLs Phosphatidate can be converted to two precursors: - diacylglycerol (precursor for TGs and neutral phospholipids) - cytidine diphosphodiacylglycerol (CDPdiacylglycerol) (precursor for acidic phospholipids) Synthesis of TGs and neutral phospholipids Phosphatidylethanolamine Triacylglycerol Phosphatidylcholine Synthesis of TGs Diacylglycerol can be acylated to triacylglycerol (in adipose tissue and liver) Enzyme: acyltransferase Synthesis of neutral phospholipids CDP-choline or CDP-ethanolamine are formed from CTP by the reaction: CTP + choline phosphate CDP-choline + PPi CTP + ethanolamine phosphate CDP-ethanolamine + PPi Diacylglycerol react with CDP-choline or CDPethanolamine to form phosphatidylcholine or phosphatidylethanolamine Synthesis of acidic phospholipids Phosphatidylinositol can be converted to phosphatidylinositol 4,5-biphosphate which is the precursor of the second messenger inositol 1,4,5-triphosphate • Interconver -sions of phosphatidylethanolamine and phosphatidylserine