Mass Pycmometer + sample
advertisement

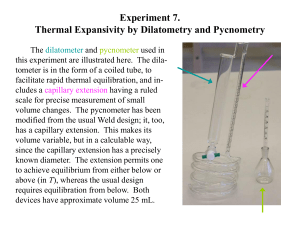
Chemistry 1A Experiment 1: Density CSUS Department of Chemistry 1 Before getting started, fill a 400 mL beaker with deionized (di) water from the center tap in the sinks. Allow this to come to room temperature, measure the temperature with your thermometer, Use this temperature to find the density of water on the chart at instructors desk. Before using the balances, be sure that you are shown the correct way to operate them. If you break it you buy it! You will work alone in this experiment. If I find data that has been shared, both parties will receive a zero score and be put on notice! 2 Experiment 1: Density •You will use different methods to measure the density of a liquid and solid in this experiment. •Each method has different precisions of measurement. •You will need to keep this in mind when recording your data. Part A: Density of a liquid Graduated Cylinder Method: Choose a liquid from the hood, record its identity. Volume: Mass: Waste: Read to ± 0.1 mL Read to ± 0.01g (top loading balance) Dispose of the waste in the Organic waste jug in the waste hood. 3 Part A: Density of a liquid Pycnometric Method: A “pycnometer” is a flask, usually made of glass, with a close-fitting ground glass stopper so that air bubbles may escape from the apparatus. This enables the density of a fluid to be measured precisely accurately. You will need to check out your pycnometer from the stockroom. Step 1: Rinse and dry your pycnometer before using. Calibrate the flasks volume using water, wipe all excess water off of the pycnometer before weighing! All masses are recorded to ± 0.0001g using the analytical balance. Step 2: Using the same liquid as before, determine the density with your calibrated pycnometer. Dispose of the waste in the organic jug in the waste hood. 4 Mass of water contained by the pycnometer using density Yields volume of water contained by the pycnometer Volume of the pycnometer 5 Part B: Density of a Solid Obtain a solid sample from your the front of the room. Use the larger of the two samples. Record the identity. Part1: Direct volume calculation (Larger of the two samples) Mass: Read to ± 0.01g (top loading balance) Ruler: Read to ± 0.1cm (1mm) Part 2: Water Displacement Using the mass from part 1, determine the volume of your solid via water displacement. Volume: Read to ± 0.5 mL 6 The level of the liquid rises due to displacement. The difference in volume is the volume of the object. 8.5 mL 5.0 mL All volumes using this method must be reported to 0.5 mL. 3.0 mL 8.5 mL The volume is accurate because the liquid fills in completely around the irregular shape! 5.0 mL 7 Part 3: Pycnometric Method: •Using the smaller of the two metal samples, determine the volume via your calibrated pycnometer. •Place the solid in your pycnometer and weight to ± 0.0001g on the analytical balance. •Fill the pycnometer with water making sure there are now bubbles when the stopper is replaced. Record the mass. Mass Pycnometer + sample + water - Mass Pycmometer + sample Mass water surrounding the sample Mass water surrounding the sample Volume of water surrounding the sample Volume of sample Density of sample 8 Mass Pycnometer + sample + water - Mass Pycmometer + sample Mass water surrounding the sample Mass water surrounding the sample Volume of water surrounding the sample using density Volume of sample Subtract the volume of water from the total pycnometer volume Density of sample using the sample mass 9 •Goggles on at all times •Only water goes in the sink, all other waste in the hood •check out and return the pycnometers from the stock room. DO NOT LEAVE THEM IN YOUR LOCKER! • Return the rulers and solid samples to the front of the room. •Pick up any paper towels and put them in the trash. •Wipe down any spills on the bench tops. 10