Density of Pennies Lab
advertisement

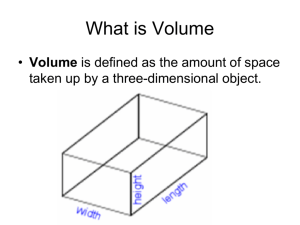
Measuring the Density of Pennies Introduction: Todays penny is quite different from the penny of 40 years ago. Before 1982, pennies were made of an alloy of copper. Since then, they have been made with an ouside coating of copper and an inner core of a different metal. These differences in the composition of older and more recently minted pennies have resulted in differences in the penny’s characteristics, including its density (mass per unit of volume). In this experiment, you will determine and compare the densities of pennies minted before 1982 and after 1983, and use density data to try to identify the metal used in the core of pennies minted after 1983. Objectives: 1. Determine the densities of pennies minted before 1982 and after 1983. 2. Try to identify the metal in the core of pennies minted after 1983 by using their density. Materials: - 25 pre-1982 pennies (including 1982) 25 post-1983 pennies Scale - 50mL graduated cylinder - Paper Towels Procedure: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Choose a set of pennies to work with. Either 25 pre-1982 pennies or 25 post-1093 pennies. Don’t trust the beakers. Actually look at the dates on the pennies. Find the mass of 5 pennies that you chose. Record the mass in the data table below. Add 5 more pennies to the first group and obtain the mass of these 10 pennies. Record the mass. Repeat step 2, each time adding 5 more pennies to those already on the balance, until you have used 25 pennies. Fill a 50-mL graduated cylinder to about the 20mL mark. Record the exact volume of water in the graduated cylinder. (remember to look at the bottom of the meniscus, I expect that you won’t have exactly 20 mL) Using the same set of pennies from steps 1-4, tilt you graduated cylinder and gently slide 5 of the pennies into the graduated cylinder. Record the new total volume in the data table. Add 5 more pennies to the graduated cylinder, making a total of 10 pennies. Record the water level in the table. Add 5 more pennies ot the cylinder and record the total volume. Repeat step 8 until you have added all 25 pennies of the set into the cylinder. Discard the water from the graduated cylinder. Use 1 paper towel to dry the pennies and return all the pennies to the correct beaker. 11. Repeat steps 1-10 with a set of 25 pennies from the other set of pennies (either pre-1982 or post-1983. Data Tables: Table 1. Data for Pre-1982 pennies Number of Pennies Mass (g) 0 Total Volume in graduated Cylinder (mL) Net volume of Pennies (mL) Density of pennies (g/mL) xxxxxxxxxxxxxxx xxxxxxxxxxxxxxx Xxxxxxxxxxxxx xxxxxxxxxxxxx Xxxxxxxxxxxx xxxxxxxxxxxx Xxxxxxxxxxxxxxxxxx Xxxxxxxxxxxxxxxxx Xxxxxxxxxxxxxxxxx Xxxxxxxxxxxxxxxxx Average Density 5 10 15 20 25 Xxxxxxxxxxxxx Xxxxxxxxxxxxx Table 2: Data for Post-1983 pennies Number of Pennies Mass (g) 0 otal Volume in graduated Cylinder (mL) Net volume of Pennies (mL) Density of pennies (g/mL) xxxxxxxxxxxxxxx xxxxxxxxxxxxxxx Xxxxxxxxxxxxx xxxxxxxxxxxxx Xxxxxxxxxxxx xxxxxxxxxxxx Xxxxxxxxxxxxxxxxxx Xxxxxxxxxxxxxxxxx Xxxxxxxxxxxxxxxxx Xxxxxxxxxxxxxxxxx Average Density 5 10 15 20 25 Xxxxxxxxxxxxx Xxxxxxxxxxxxx Conclusions: 1. Using the computers in the back of the room, go to http://www.science.co.il/PTelements.asp?s=Density and find the density of copper. Compare this to the average density of you pre-1982 pennies. What is the % error of your findings? % Error = 𝑬𝒙𝒑𝒆𝒓𝒊𝒎𝒆𝒏𝒕𝒂𝒍 𝒗𝒂𝒍𝒖𝒆−𝑹𝒆𝒂𝒍 𝑽𝒂𝒍𝒖𝒆 𝑹𝒆𝒂𝒍 𝑽𝒂𝒍𝒖𝒆 Real value is what you find on website. If your percent error I negative, simply remove the negative sign. 2. What are two things in the procedure of this lab that could have cause error in you findings? 3. Find element on the same websites that have similar densities to you post-1983 pennies. List the 3 closest metals. Requirements for density of pennies lab Report (general chem.) All parts of this report must be typed. Title: Purpose: Procedure: Data: (2tables, one for old pennies one for new just like the tables in you lab procedure. Conclusions: (http://www.science.co.il/PTelements.asp?s=Density) 1. State the density of copper, the average density of the pre-83 pennies and the percent error. 2. State the average density of the post-83 pennies and possible metals that it might be (Use the chart on the website listed above). 3. State possible sources of error