3.4
Density
>
Determining Density
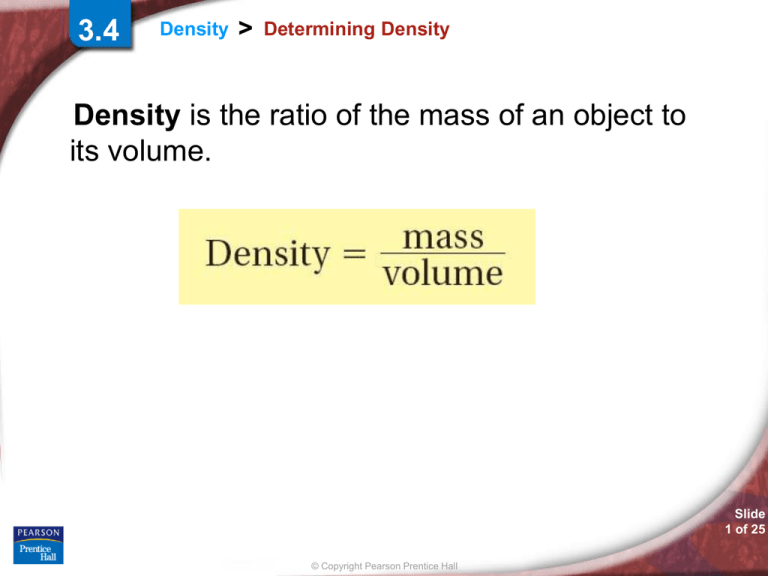
Density is the ratio of the mass of an object to
its volume.
Slide
1 of 25
© Copyright Pearson Prentice Hall
3.4
Density
>
Determining Density
Density is an intensive property that
depends only on the composition of a
substance, not on the size of the
sample.
Slide
2 of 25
© Copyright Pearson Prentice Hall
Density
>
So, the density for a given substance
defines the relationship between mass
and volume
Like any relationship, it gives you 2
conversion factors
For gold,
19.3 g/cm3 and 1 cm3/19.3 g
Slide
3 of 25
© Copyright Pearson Prentice Hall
3.4
Density
>
Determining Density
The density of corn oil is
less than the density of
corn syrup. For that
reason, the oil floats on
top of the syrup.
Slide
4 of 25
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 46
A student finds a shiny piece of metal that she thinks
is aluminum. In the lab, she determines the metal
has a volume of 245 cm3 and a mass of 612 g. Is
this metal aluminum?
Slide
5 of 25
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM
Well, we can find the density of the material and
compare it to the density in the table for aluminum
and see if they match.
612 g
245 cm3
=
2.50 g
cm3
From the table, the density of aluminum is 2.70
g/cm3, so no the metal is not pure aluminum
Slide
6 of 25
© Copyright Pearson Prentice Hall
3.4
Density
>
Density and Temperature
Experiments show that the volume of most
substances increases as the temperature
increases. Meanwhile, the mass remains
the same. Thus, the density must change.
The density of a substance generally
decreases as its temperature
increases.
Slide
7 of 25
© Copyright Pearson Prentice Hall
Practice Problems for Sample Problem 3.10
Problem Solving 3.47 Solve
Problem 47 with the help of an
interactive guided tutorial.
Slide
8 of 25
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 3.11
Slide
9 of 25
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 3.11
Slide
10 of 25
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 3.11
Slide
11 of 25
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 3.11
Slide
12 of 25
© Copyright Pearson Prentice Hall
Practice Problems for Sample Problem 3.11
Problem Solving 3.48 Solve
Problem 48 with the help of an
interactive guided tutorial.
Slide
13 of 25
© Copyright Pearson Prentice Hall
3.4 Section Quiz
1. If 50.0 mL of corn syrup have a mass of 68.7
g, the density of the corn syrup is
a. 0.737 g/mL.
b. 0.727 g/mL.
c. 1.36 g/mL.
d. 1.37 g/mL.
Slide
14 of 25
© Copyright Pearson Prentice Hall
3.4 Section Quiz
2. What is the volume of a pure gold coin that
has a mass of 38.6 g? The density of gold is
19.3 g/cm3.
a. 0.500 cm3
b. 2.00 cm3
c. 38.6 cm3
d. 745 cm3
Slide
15 of 25
© Copyright Pearson Prentice Hall
3.4 Section Quiz
3. As the temperature increases, the density of
most substances
a. increases.
b. decreases.
c. remains the same.
d. increases at first and then decreases.
Slide
16 of 25
© Copyright Pearson Prentice Hall
Slide
17 of 25
© Copyright Pearson Prentice Hall