Photoperiodism
advertisement

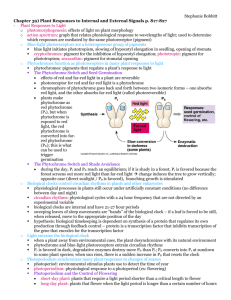
Photoperiodism Photoperiodism Photoperiodism: • It is mechanism in plants in relation to light. Both plants and animals are able to detect length of day and night and respond to it. Definitions: • i) It is the response of plant to relative length of day and night i.e. light and dark period. • ii) It is the response of plant to the timing of light and darkness • iii) The response of plant to the length of day which enable and organism to adapt to seasonal changes in its environment. First experimental study • Garner and Allard (1920) grew Maryland mammoth variety of tobacco (Nicotiana tabaccum) and Biloxi cultivar of soybean (Glycine max) • Both the plants were grown in high light intensity or light period, they did not flower. In summer, during long day length, these two species were growth in field and both the varieties did not flower but some of the plants grown in pots were transferred in a hut near the field (shade or darkness). The plants of field did not flower but the plants placed in hut started flowering at the same time. Both the plants were short day plants because both required short photoperiod or short day length. It reveals from the experiment that plants definitely possess response to light period. Conclusion • Garner and Allard concluded that the length of the day controlled flowering. This conclusion was supported by the fact that mutant could be kept in vegetative state during the winter months by merely lengthening the days with artificial light. • Garner and Allard termed the response of maryland mammoth and biloxi to day length as “photoperiodism”. Classification of plants according to photoperiodic reactions • Photoperiodic response • Any response by a plant to the duration and order of sequence of light and dark periods may be called as photoperiodic response • On the basis of photoperiodic response plants are of following types • 1. Long day plants (LDPs) • These plants flower when the day length is higher than the critical day length • There are two categories • a) Qualitative LDPs • b) Quantitative LDPs • a) Qualitative LDPs • The plants which specifically require long days for flowering. If no long day, they will not flower e.g. • Beta vulgaris (sugar beet) • Spinacea oleracea (spinach) • Raphanus sativus (Raddish) • Hyocyamus niger (Ajwain) • Hordeum vulgare (barley) • b) Quantitative LDPs • Those plants in which flowering is promoted by long days e.g. • Riccinus communis (castor) • Triticum aestivum • Pisum sativum (Pea) • 2. Short day plants (SDPs) • These plants flower when the day length is less than the critical day length • A) Qualitative SDPs • Those plants that specifically require short day for flowering e.g. • Xanthium • Chrysanthemum • Nicotiana tabaccum • Zea mays • Glycine max • • • • • • • • • • • • • • • • • B) Quantitative SDPs Those plants in which flowering is promoted by short days e.g. Helianthus annuus (sunflower) Saccharum officinarum (sugarcane) Gossypium hirsutum (cotton) 3. Day neutral plants (DNPs) Those plants which do not respond to photoperiods e.g. Oryza sativa (rice) Cucumber Zea mays (some varieties) Tomato 4. Short long day plants (SLDPs) These plants require short days followed by long days for flowering. They flower in late spring or early summer e.g. Trifolium alexendrinum T. pratense (Red clover) Some varieties of wheat These plants not flower when kept in continuous short or long day periods • 5. Long short day plants (LSDPs) • These plants require long days followed by short days. They flower in late summer or autumn e.g. • Bryophyllum (pather chut) • Cestrum nocturnum • Certrum diurnum • 6. Intermediate day plants (IDPs) • Plants in which flowering neither occur in short days nor long days e.g. • Chenopodium album (bathoo) • Saccharum spontaneum (species of sugarcane i.e. surkunda kai) • Coleus hybrida • 7. Amphiphotoperiodic plants • Plants in which flowering is inhibited by intermediate day length e.g. • Media elegans (tarweed) • Setaria verticillata Critical day length or photoperiod • The light period which is essentially required to induce flowering in plants known as critical photoperiod e.g. • Hyocyamus niger which is long day plant requires photoperiod greater than 11 hours • Xanthium is a short day plant and requires photoperiod less than 15.5 hours • Long day Hyocyamus plant will start flowering only if critical photoperiod is 11 or greater than 11hours • Short day plant Xanthium will start flowering when light period will be less than 15.5 hours • If light period is 14 hours then both plants will flower Photoinductive cycle • Photoperiod induction means that plant need to experience favorable photoperiod for few days then they will flower even if they are placed in unfavorable day length • Favorable photoperiod cause the change of state in the leaves which is called photoperiodic induction • For both SDP and LDPs, definite number of such cycles are required for flowering to occurs and this is know as photoinductive cycle • Number of photoinductive cycles apply to a plant is very important because they control the number of flowers and their maturation Continuo---• For example, Xanthium a short day plant, when we apply one short day cycle its flower will take 64 days to mature i.e. late flowering and late maturation • In continues short day condition, it will take 13 days to mature • Short day plant cocklebur grown under long days one cycle of short day and long night return to long days • Flower after 64 days Mechanism of photoperiodism • This process is involved following steps i.e. general principles • 1. Perception: by leaves • 2. Transmission: Stimulus from leaf to shoot apex • 3. Evocation: occurance of flowering at apex • There are two important aspects • 1. phytochrome is a photo or light receptor because e it receives light • It is a chemical or pigment that receives light • 2. stimulus: some scientists say that it’s a florigen produced in leaves and transmitted to stem apex and causes flowering (it is hypothetical substance) Vernalization • It is a process in which flowering is promoted by a cold treatment given to fully hydrated seeds or growing plants • Plants that require cold treatment • Plants that require vernalization show delayed flowering or even remain vegetative or show rosette like growth if they are not exposed to cold treatment during germination • Range of vernalization temperature • The vernalization temperature is just below 0 to 10 C but the most effective and precise temp range is 1-7 °C depending on species Continuo ---• The effect of cold treatment increased with increasing duration of cold treatment until the response is saturated • Response usually requires several weeks of exposure to low temp but precise duration varies from species to species • Vernalization appears to take place primarily in shoot apical meristem • If stem apex is chilled, flowering will occur Vernalization may involve epigenic changes in gene expression • Epigenic regulation: changes in gene expression that are stable even after the removal of signal that induced changes. • Winter-annual ecotypes of Arabidopsis that require long days and vernalization for flowering, a gene (FLC) that acts as repressor of flowering has been identified (FLC) • FLOWERING LOCUS (FLC): highly expressed in nonvernalized shoot apical meristems (Michaels and Amasino, 2000). • After vernalization this gene is epigenetically switched off by an unknown mechanism in rest of life cycle, permitting flowering to occur in response to long days. • In the next generation, however, the gene is switched on again. Phytochromes • Phytochrome is a pigment that absorbs light (red and far-red) which is effective in causing photomorphogenesis in plants. It is ubiquitous chromoprotein which plays a significant role in almost every stage of plant development • Photomorphogenesis: the control of morphogenesis or development by light is called photomorphogenesis. Discovery • Garner and Allard (1920) work led to the discovery of the pigments responsible for the absorption of light. Most researvh leading to phytochrome detection and isolation was accomplished in Maryland (USA) between 19451960. • I) H. A. Borthwick (1972): He summarized the history of discovery of phytochrome. • II) Briggs (1976) • III) S. B. Hendricks (1970): Briefly described the discovery of phytochromes Continuo ---• The initial observations were on lettuce (salad plants) seed. The germination of lettuce seeds is stimulated by red light and inhibited by far-red light. After many years, further research shows that 100% germination in seeds occurs that received red light as final treatment. However, germination was strongly inhibited by far-red treatment (Flint, 1936). On the basis of these observations, there were two possibilities, one is “there are two pigments, one pigment is red-light absorbing and other is far-red light and two pigments act antagonistically or there might be a single pigment that can exist in two interconvertable forms, a red light absorbing and a far-red light absorbing form (Borthwick, 1952). Buttler et al. (1959) demonstrated phytochrome properties in vitro from plant extracts. Types of Phytochromes • In plants, phytochromes are of 5 different types • Phytochrome A • Phytochrome B • Phytochrome C • Phytochrome D • Phytochrome E Physical properties of phytochrome • Absorption spectrum: Different types of pigments absorb the different properties of visible spectrum of radiant energy and %age of each wavelength of light absorbed by the pigment is known as absorption spectrum. • Action spectrum: It can be defined as the relative effectiveness of different wavelengths of light on photomorphogenesis Phytochrome can interconvert between Pr and Pfr forms • In dark or etiolated plants, phytochrome is present in a red-light absorbing form, referred to as Pr because it is synthesized in this form. It is then converted to Pfr form by red light. This far-red absorbing Pfr form again converted to Pr by far-red light. This convertion/reconversion property is the most distinctive property of phytochrome • Red-light (660 nm) • Pr Pfr • Far-red (730 nm) • The proportion of Pfr form after saturation by red light is only about 85%, whereas very small amount of far-red light absorbed by Pr makes it impossible to convert Pfr entirely to Pr by broadspectrum far-red light. • Instead, an equilibrium of 97% Pr and 3% Pfr is achieved. This equilibrium is termed as the photostationary state. • Pfr is physiologically active form of phytochrome • Comparison between Pr and Pfr form Pr Pfr First synthesized Pr is converted into Pfr Inactive form Active form Stable form Unstable form Blue color Olive green Cis form COOH COOH Trans form COOH COOH Chemical properties of phytochromes • Phytochrome is a soluble protein with a molecular mass of about 250 kDa. It is a dimer made up of two equivalent subunits. Each subunit consists of two components, one is prosthetic group which is light absorbing pigment molecule called chromophore and a polypeptide chain called the Apoprotein. The apoprotein monomer has a molecular mass of about 125 kDa. Together, apoprotein and its chromophore make up the holoprotein. In higher plants the chromophore of phytochrome is a linear tetrapyrrole termed as phytochromobilin. The apoprotein and chromophore are attached through cystein residue. Synthesis of phytochrome • Phytochromobilin is synthesized in plastids and released into the cytosol, where it binds with phytochrome apoprotein • Active – play role in flowering Red • Synthesis Pr Pfr Biological Far-red response Dark or thermal conversion Distribution among species • In addition to angiosperms, phytochromes are also found in gymnosperms, liverworts, mosses, ferns and some green algae suggesting it might be found in all photosynthetic organisms except photosynthetic bacteria. Little is known about chemical properties of phytochrome in species other than angiosperms. Distribution among tissues and cells • Phytochromes are present in most organs of plants including roots. In etiolated seedling the highest phytochrome levels are usually found in meristematic regions such as bud and first node. Phytochromes are most abundant in young, undifferentiated tissues. • Those cells where mRNA are most abundant, phytochromes are found. There is a strong correlation between the abundance of phytochrome and potential of development in cells which shows that phytochromes play an important role in controlling such developmental changes. Role of phytochromes in biological processes • Phytochrome mediated effects can be grouped into three categories on the basis of their energy requirements. • i) Very Low Fluence Responses (VLFRs) • ii) Low Fluence Responses (LFRs) • iii) High Irradiance Reactions (HIRs) • Fluence: Moles of quanta per unit area (mol m-2) • Fluence rate or Irradiance: The moles of quanta per unit area per second. i) Very Low Fluence Responses (VLFRs) • These are not photo-reversible because these responses become saturated at very low light levels i.e. that levels which are below those that cause a measureable conversion of Pr to Pfr. ii) Low Fluence Responses (LFRs) • LFRs include the photoreversible phytochrome responses such as seed germination and de-etiolation. LFRs convey information to seeds about its position relative to soil surface and increase the potential for seedling to become established in light and initiate photosynthesis before nutreint reserves of the seedling are exhausted. iii) High Irradiance Reactions (HIRs) • These are also not photoreversible. HIRs require prolonged exposure to high irradiance and are time dependent • Morhphogenetic responses i) VLFRs: Some phytochrome responses can be initiated by fluence as low as 0.0001 µmol m-2 (on tenth of the amount of light emitted from a briefly in a single flash) and they saturates at about 0.05 µmol m-2 e.g. in darkgrown oat seedlings, red light can stimulate the growth of coleoptile at such low fluences. Arabidopsis seeds can be induced to germinate with the red light in the range of 0.001 to 0.1 µmol m-2. ii) LFRs: This set of phytochrome responses can not be initiated until the fulence reaches 1.0 µmol m-2 and they saturates at 1000 µmol m-2. they include most of the red/far-red photoreversible responses such as promotion of lettuce seed germination and regulation of leaf movement. iii) HIRs: a) Synthesis of anthocyanins in various dicot seedling and apple skin segments b) Inhibition of hypocotyl elongation in mustard, lettuce and petunia seedling c) Induction of flowering in henbane (Hyoscyamus) d) Plumular hook opening in mustard e) Production of ethylene in sorhgum Ecological role phytochromes Phytochromes enable plants to adapt to changing light conditions Phytochromes regulate the sleep movements of leaves Floral meristems and floral organ development • Floral meristems: Floral meristems can be distinguished from vegetative meristems, even in the early stages of reproductive development by their larger size. The transition from vegetative to reproductive development is marked by an increase in cell division within the central zone of shoot apical meristem. In vegetative meristem, the cells of central zone slowly complete their division cycles. As reproductive development starts, the increase in size of meristem is largely as a result of increased division rate of cells • Most of the genetic and molecular studies have been done on • i) Arabidopsis • ii) Antirrhinum (Snapdragon) • During vegetative growth of Arabidopsis vegetative apical meristem produces phytomeres (phytomere consists of a leaf, the node to which leaf is attached, the axillary bud and the internode below the node) with very shot internodes, resulting in the basal rosette of leaves. • As reproductive development starts, the vegetative meristem is transformed into an inderminate primary inflorescence meristem that produces floral meristems on its flanks. The lateral bud of cauline leaves develop into secondary inflorescence meristem and their activity repeats the pattern of development of primary inflorescence meristem. Floral organ development • The four different types of floral organs are intiated as separate whorls. These organs are i) sepals ii) petals iii) stamens and iv) carpels. The initiation of innermost organs, the carpels, consumes all the meristematic cells in the apical dome. • In wild type Arabidopsis flower, the whorls are arranges as follows: 1. The outermost whorl consists of four sepals 2. The second whorl consists of four petals 3. The 3rd whorl consists of six stamens, two shorter and four larger 4. The 4th whorl is a single complex organ, the gynoecium, which is composed of an ovary with two fused carpels. Three classes of genes regulate floral development • There are three classes of genes that regulate floral development • I) Floral organ identity genes • II) Cadastral genes • III) Meristem identity genes • I) Floral organ identity genes: These genes directly control floral identity. The proteins encoded by these genes are transcription factors that likely control the expression of other genes whose products are involved in the formation of floral organs. • II) Cadastral genes: These genes act as regulators of the floral organ identity genes by setting boundaries for their expression. • III) Meristem identity genes: These genes are necessary for the initial induction of the organ identity genes Floral organ identity genes • Homeotic genes: First discovered in Drosophila. These genes regulate the location of body parts in flies. • The floral organ identity genes were identified through homeotic mutations that altered the place of floral organs e.g. Arabidopsis plants with mutations in the APETALA2 (AP2) gene produce flowers with carpels at the place of sepals and stamens at the position of petals. Three classes of homeotic genes control floral organ identity • Five different genes are known to specify floral organ identity in Arabidopsis • APETALA1 (AP1) • APETALA2 (AP2) • APETALA3 (AP3) • PISTILLATA (PI) • AGAMOUS (AG) • The homeotic genes fall into three classes/types depending on three different activities i.e. type A, B and C activities • Type A activity: Type A activity is encoded by AP1 and AP2 control organ identity in the first and second whorls. Loss of type A activity results in the formation of carpels instead of sepals in first whorl and of stamens instead of petals in second whorl. • Type B activity: This activity is encoded by AP3 and PI control organ determination in the second and 3rd whorls. Loss of type B activity results in the formation of sepals instead of petals and carpels instead of stamens. • Type C activity: This activity is encoded by AG, controls events in the 3rd and 4th whorls. Loss of type C activity results in the formation of petals instead of stamens and sepals instead of carpels. ABC model • • • • • • • • ABC model was studied in i) Arabidopsis ii) Antirrhinum (Snapdragon) Cells in which only A gene is present or A gene expresses develop into sepals Cells in which both A and B genes are expressed develop into petals Cells in which both B and C genes are expressed develop into stamens Expression of C gene alone turns on the development of carpel Genes are present in each cell of the whorl. Their expression is specific.