Nutrient Cycles - lapazcolegio2015-2016
advertisement

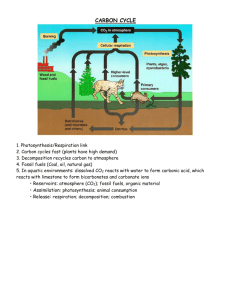
Biogeochemical Cycles Nutrient Cycles What are nutrients? Nutrients are chemicals that organisms must take in from their environment Macronutrients are required by organisms in large amounts and include water, carbon, hydrogen, oxygen, nitrogen, phosphorus, sulfur, and calcium Micronutrients are required in trace amounts and include zinc, molybdenum, iron, selenium, and iodine Why do we need to understand nutrient cycles? Nutrients do not flow down onto Earth in a steady stream from above (like energy) describe the pathways nutrients follow between communities and the nonliving portions of ecosystems Reservoirs are sources and storage sites of nutrients Major reservoirs are usually in the abiotic environment (rocks, water, atmosphere) Processes are the changes that occur to move nutrients May involve changes of state or chemical form Hydrologic (water) cycle Water is essential for all terrestrial communities because other nutrients must be dissolved in it before they can be used major reservoir in the oceans travels through the atmosphere, to reservoirs in freshwater lakes, rivers, and groundwater, and then back again to the oceans The oceans contain more than 97% of Earth’s water Solar energy evaporates water, and it comes back to Earth as precipitation The Hydrologic Cycle reservoirs processes water vapor in the atmosphere precipitation over land precipitation over the ocean evaporation from land and transpiration from plants evaporation from the ocean evaporation from lakes and rivers lakes and rivers seepage into soil groundwater, including aquifers runoff from rivers and land extraction for agriculture water in the ocean carbon cycle Chains of carbon atoms form the framework of all organic molecules, the building blocks of life major reservoirs in the atmosphere and oceans Moves through producers and into the bodies of consumers and detritus feeders, and then back to its reservoirs The Carbon Cycle reservoirs CO2 in the atmosphere processes trophic levels burning fossil fuels CO2 dissolved in the ocean respiration fire photosynthesis producers consumers detritus feeders and decomposers decomposition fossil fuels (coal, oil, natural gas) nitrogen cycle Nitrogen is a crucial component of proteins, many vitamins, nucleotides (such as ATP), and nucleic acids major reservoir in the atmosphere reservoirs of ammonia and nitrate in the soil and water, through producers and into consumers and detritus feeders, and then back again to its reservoirs nitrogen gas (N2) makes up 78% of the atmosphere, this form of nitrogen cannot be utilized by plants Plants utilize nitrate (NO3–) or ammonia (NH3) as their nitrogen source N2 is converted to ammonia by specific bacteria during a process called nitrogen fixation Denitrifying bacteria break down nitrate, releasing N2 back to the atmosphere The Nitrogen Cycle reservoirs processes trophic levels N2 in the atmosphere burning fossil fuels lightning application of manufactured fertilizer consumers ammonia and nitrates in water producers uptake by producers detritus feeders and decomposers nitrogen-fixing bacteria in soil and legume roots decomposition denitrifying bacteria ammonia and nitrates in soil phosphorus cycle Phosphorus is a crucial component of ATP and NADP, nucleic acids, and phospholipids of cell membranes; it is also a major component of vertebrate teeth and bones major reservoir in rock bound to oxygen as phosphate moves from phosphate-rich rocks to reservoirs of phosphate in soil and water, through producers and into consumers and detritus feeders, and then back to its reservoirs The Phosphorus Cycle reservoirs processes trophic levels phosphate in rock geological uplift application of manufactured fertilizer runoff from rivers consumers producers detritus feeders and decomposers decomposition runoff from fertilized fields uptake by producers phosphate in water phosphate in soil phosphate in sediment formation of phosphate-containing rock Human Disruption Many of the environmental problems that plague modern society are caused by human disruption of biogeochemical cycles. industrial processes transfer toxic substances such as lead, arsenic, mercury, uranium, and oil into the environment bioaccumulation addition of herbicides and pesticides to lawns and shrubs farm fields, gardens, and suburban lawns, ammonia, nitrate, and phosphate are supplied by chemical fertilizers combustion of fossil fuels releases sulfur dioxide and nitrogen oxides into the atmosphere eutrophication – adding nutrients to water acid rain Carbon emmisions release CO2 Enhanced global warming