spirometry reference values
advertisement

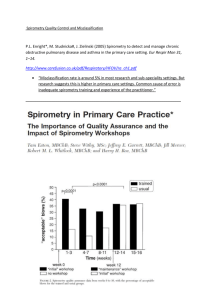
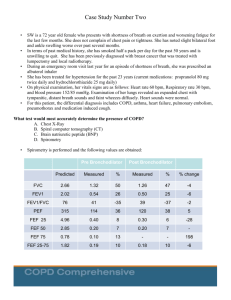
SPIROMETRY REFERENCE VALUES: - A SYSTEMATIC REVIEW OF PUBLISHED ARTICLES FROM 1998 TO 2008 - CLASS 1 Regent: Altamiro Pereira, MD PhD Adviser: Tiago Jacinto, MD INTRODUCTION TO MEDICINE 2009/2010 ii. Are they different methodological quality? in terms of Research Questions i. How many and how good are the studies published in the last decade? 4 Hughes, 2007 Reference values provide a point of reference to help to interpret and determine a patient’s condition: a test result is normal if it falls within the range predicted for the age, sex and height of the patient, based on large population studies 4 performing a test, in order to avoid misdiagnosis; Reference values are important statistical parameters to obtain medical relevant information Introduction It is crucial to have adequate reference values when Spirometry is an exam that allows to assess a patient’s pulmonary function using a spirometer: The spirometer measures the volume and flow of the forced exhaled air during a period of time 2 Two reference values are commonly used: the FVC (total amount of exhaled air) and FEV1 (exhaled air during the first second) 6,7 2 Enright, n.d. Kuster, 2008 8 Miller, 2007 6 Different People have Different Values Pre-calculated values Inappropriate reference values can lead to bad diagnostics 1,3,5,6 SYSTEMATIC REVIEW AND QUALITY ASSESSMENT 1 3 Baur, 1999 Garcia-Rio, 2004 5 Ip, 2006 6 Kuster, 2008 9 STROBE statement Aims i. Summarize data published in the last decade; ii. Analyze and rate the obtained information using the 9 STROBE checklist ; iii. Propose the most suitable values for Portugal or suggest the conduction of a study Methods Study design: Systematic review; Key words: Systematic review, Spirometry, Reference Values, Forced Expiratory Volume, Forced Vital Capacity Procedure: 1. Search for articles 2. Select and layout articles that meet the criteria 3. Rate using the STROBE checklist 4. Conclusion 7 Liberati, 2009 General Methods Data Sources (online databases): Inclusion Criteria Exclusion Criteria o Articles whose full text can not be achieved for free; o Study subjects with occupational exposure to inhalable materials; Articles that calculate new FEV1 and FVC spirometric reference values o Articles about studies with missing data (reference equations, FEV1 or FVC values); o Study subjects with history of pulmonary disease, smoking or current disease; Published from 1998 to 2008 o Articles in languages other than Portuguese or English; o Articles that don’t refer the equipment used; o Participants under 18 years old; o Not cross-sectional; Criteria o Studies conducted in animals; QUERY APPLICATION ON MARCH 5TH, 2010 ONLINE DATABASE QUERY USED: ARTICLES FOUND (N) ("1998"[Publication Date] : "2008"[Publication Date]) AND ("Spirometry"[tiab] AND ("Reference values"[mh] OR "Reference equations")) 218 814 (TITLE-ABS-KEY(spirometry) AND TITLE-ABS-KEY(“reference values” OR “reference equations” OR “normal values” OR “normative values”)) AND (LIMIT-TO(PUBYEAR, 2008) OR LIMIT-TO(PUBYEAR, 2007) OR LIMITTO(PUBYEAR, 2006) OR LIMIT-TO(PUBYEAR, 2005) OR LIMIT-TO(PUBYEAR, 2004) OR LIMIT-TO(PUBYEAR, 2003) OR LIMIT-TO(PUBYEAR, 2002) OR LIMIT-TO(PUBYEAR, 2001) OR LIMIT-TO(PUBYEAR, 2000) OR LIMITTO(PUBYEAR, 1999) OR LIMIT-TO(PUBYEAR, 1998)) 276 Schematic representation of our work plan Start Search Read title by two reviewers considering the inclusion criteria Both approve? Yes Include article No Read abstract by two reviewers considering the exclusion criteria Fulfills the inclusion criteria? No No Both approve? Yes Yes Read abstract by a “third reviwer” Exclude article Include article Include article Exclude article Yes Fulfills any exclusion criteria? Read full text by two reviewers Extract variables and score using STROBE checklist Finish No Include article Research Method Read title by a “third reviewer” 2. Select and layout articles that meet the criteria Procedure 1. Search for articles Modified STROBE check-list (a) Indicate the study’s design with a commonly used term in the title or the abstract Title and abstract 1 (b) Provide in the abstract an informative and balanced summary of what was done and what was found Introduction Background/ rationale 2 Explain the scientific background and rationale for the investigation being reported Objectives 3 State specific objectives, including any prespecified hypotheses Methods Study design 4 Present key elements of study design early in the paper Setting 5 Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection Participants 6 (a) Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls (b) For matched studies, give matching criteria and the number of controls per case NA 7 Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable NA Data sources/ measurement 8* For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group Bias 9 Describe any efforts to address potential sources of bias Study size 10 Explain how the study size was arrived at Quantitative variables 11 Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why Variables (a) Describe all statistical methods, including those used to control for confounding (b) Describe any methods used to examine subgroups and interactions Statistical methods (c) Explain how missing data were addressed 12 (d) If applicable, explain how matching of cases and controls was addressed (e) Describe any sensitivity analyses NA Results Participants 13 (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed *(b) Give reasons for non-participation at each stage (c) Consider use of a flow diagram Descriptive data Outcome data (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders 14 * (b) Indicate number of participants with missing data for each variable of interest 15 Report numbers in each exposure category, or summary measures of exposure * (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included Main results 16 (b) Report category boundaries when continuous variables were categorized (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period Other analyses 17 Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses Discussion Key results 18 Summarise key results with reference to study objectives 19 Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias Interpretation 20 Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence Generalisabilit y 21 Discuss the generalisability (external validity) of the study results Limitations Other information Funding 22 Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based Title Year Smolej, N. et al, New reference equations for forced spirometry in elderly persons 2008 0,93 2001 0,88 M. Susan Marion et al. A. Langhammer et al. M.H. Boskabadya et al. Spirometry Reference Values for American Indian Adults: Results From the Strong Heart Study Forced spirometry reference values for Norwegian adults: the Bronchial Obstructi Lung Function Values in Healthy Non-Smoking Urban Adults in ATS/ERS criteria Spirometer Number of Participants Age Range Croatia Yes Jager’s Pneumoscreen, Wurtzburg, Germany 261 (154/1079 65-86 United States of America Yes Mijnhart S&M Instruments 3167 (1972/1195) 45-74 908 (546/362) 20-80 Score Country 2001 0,75 Norway Yes MasterScope spirometer, version 4.15, Erich Jaeger GmbH, 2002 0,72 Iran Yes Model ST90, Fukuda Sangyo 572 (246/326) 18-65 627 (327/300) 20-80 Gutierrez, Carlos et al. Reference values of pulmonary function tests for Canadian caucasians 2004 0,69 Canada Yes Morgan Model USA System; Med Science wedge spirometer model 570, PK Morgan Rolling seal spirometer, Stead wells spirometer Marsh, S. et al. Complete reference ranges for pulmonary function tests from a single population 2006 0,69 New Zealand Yes Jaegar Master Screen Body 266 (156/110) 18-70 12898 (6419/6479) 20-44 Roca, J. et al. References values for forced spirometry 1998 0,67 England No Sensor Medics 2130; Jaeger Pneumolab Vitalograph; Morgan Spirograph DS12; Sensor Medics Pne 12050; Spirotech S500; Hewlett Packard Falaschetti, E. et al. Prediction equations for normal and low lung function from the Health Survey for 2004 0,6 England Yes Vitalograph Escort Spirometer 3053 (556/2497) 16-75 Muhammad Asif Memon et al. Spirometric reference values in healthy, nonsmoking, urban Pakistani population Yes Micromedical, Microloop and Microrint (airway resistant) in Conjunction with spida 5 PC software 504 (183/321) 15-65 2007 0,6 Pakistan Variable Extraction Author Equation FEV1 Female Equation FVC Female r2 FEV1 Male Female r2 FVC Male Female RSD FEV1 Male RSD FEV1 Female RSD FVC Male -0.0200(A) + 0.0001508H) - 0.677 -0.0184(Age) + 0.0349(Height) 3.189 -0.0230(A) + 0.000130(H) 0.715 0,81 0,57 0,76 0,43 0,422 0,481 0,54 2 0,612 -0.030(A) + 0.047(H) - 2.832 -0.025(A) + 0.067(H) - 5.473 -0.024(A) + 0.049(H) - 3.335 -0.025(A) + 0.037(H) - 1.091 0,62 0,73 0,56 0,66 N.A. N.A. N.A. N.A. Exp (-12.396z2.733 ln(H)- 0.0000592 A^2) Exp (-10.556z2.342 ln(H)- 0.0000685 A^2) Exp (9.851z2.189 ln(H)0.000163A^2 + 0.007237A) Exp (-9.851z2.189 ln(H)- 0.000143 A^2 + 0.006439A) 0,6 0,72 0,63 0,68 0,12 0,13 N.A. 0,13 -0.0599(H) 0,02420(A) - 5,650 -0.0807(H) 0,0129(A)- 0,840 -0.0358(H) 0,0262 (A)1,774 -0.049(H) - 0,0258 (A) - 3.208 0,43 0,53 0,39 0,49 N.A. N.A. N.A. N.A. e^(-9.37674 + 0.00183A (0.00011A^2) + 2.10839 lnH) e^(-10.36706 + 0.00334A + (0.00011A^2) + 2.32222 lnH) e^(-8.49717 + 0.00422A + (0.00015A^2) + 1.90019 lnH) e^(-9.66999 + 0.00837A + (0.00017 A^2) + 2.14118 lnH) 0,59 0,69 0,52 0,62 N.A. N.A. N.A. N.A. 0.0514H - 0.0216A 3.955 0.0678H - 0.0147A 6.055 0.0326H 0.0253A - 1.286 0.0454H 0.0211A - 2.825 0,804 0,804 0,801 0,801 N.A N.A. N.A. N.A. -2.73+0.570.031A+4.47H -5.87+0.650.03A+6.73H -2.730.031A+4.47H -5.870.03A+6.73H 0,56 0,56 0,52 0,56 0,45 0,32 0,53 0,4 -1.440+[0.030 x Ht] + [-0.020 x Age] -0.848+[0.032 x Ht] + [-0.020 x Age] -1.866 + [0.032 x Ht] + [-0.019 x Age] -3.072 + [0.042 x Ht] + [-0.020 x Age] 0,478 0,467 0,429 0,422 N.A. N.A. N.A. N.A. 1.636 H - 0.038 A + 2.553 2.825 H - 0.050 A + 2.058 -0.034 A + 4.230 -0.042 A + 5.247 0,165 0,206 0,243 0,209 0,486 0,328 0,55 9 0,41 Equation FEV1 Male Equation FVC Male M.H. Boskaba dya et al. -0.0942(A) + 0.0319(H)-3.494 Gutierrez , Carlos et al. Author A. Langham mer et al. M. Susan Marion et al. Falaschet ti, E. et al. Marsh, S. et al. Roca, J. et al. Muhamm ad Asif Memon et al. Smolej, N. et al, r2 FEV1 r2 FVC RSD FVC Female * Few recent studies with new reference equations; Expected Results * Heterogeneous concerning the methodological quality * Expose the necessity for these kind of studies Number of Articles by Year 1 0 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 Statistical Results 2 100 90 80 70 60 50 40 30 20 10 0 Median=52% Statistical Results Bo sk ab Gu ady La tie ng rr ha ez m m M er Fa ario la sc n he t ti M ar sh Ro M ca em o Sm n ol ej % of Female Participants % of Female Participants Continent of Studies 4 2 1 0 Africa America Asia Europe Oceania Statistical Results 3 1 0,9 0,8 0,7 0,6 0,5 0,4 0,3 0,2 0,1 0 Boskabady Gutierrez Langhammer Marion Falaschetti Marsh Roca Memon Smolej Average=0,73; Range 0,6-0,93 Statistical Results Article Score Detailed Score 1. Title/abstract 2. Introduction 3. Methods 4. Results 5. Discussion 6. Funding OVERALL SCORE Gutierrez, 2004 100% 100% 63% 56% 100% 0% 69% Roca, 2008 50% 100% 72% 50% 100% 100% 67% Marsh, 2006 100% 100% 50% 60% 100% 100% 69% Memon, 2007 100% 100% 64% 30% 100% 0% 60% Falaschetti, 2004 100% 100% 73% 20% 100% 100% 60% Smolej, 2008 100% 100% 100% 80% 100% 100% 93% Laghammer, 2001 50% 50% 82% 78% 75% 0% 75% Marion, 2001 100% 50% 82% 78% 75% 0% 72% Boskabady, 2002 100% 50% 70% 83% 75% 0% 72% Average Scores 89% 83% 73% 59% 92% 33% 73% Spirometer Number of Participants Age Range Croatia Yes Jager’s Pneumoscreen, Wurtzburg, Germany 261 (154/1079 65-86 0,88 United States of America Yes Mijnhart S&M Instruments 3167 (1972/1195) 45-74 2001 0,75 Norway Yes MasterScope spirometer, version 4.15, Erich Jaeger GmbH, 908 (546/362) 20-80 2002 0,72 Iran Yes Model ST90, Fukuda Sangyo 572 (246/326) 18-65 627 (327/300) 20-80 Title Year Score Country Smolej, N. et al, New reference equations for forced spirometry in elderly persons 2008 0,93 M. Susan Marion et al. Spirometry Reference Values for American Indian Adults: Results From the Strong Heart Study 2001 A. Langhammer et al. Forced spirometry reference values for Norwegian adults: the Bronchial Obstructi M.H. Boskabadya et al. Lung Function Values in Healthy Non-Smoking Urban Adults in Gutierrez, Carlos et al. Reference values of pulmonary function tests for Canadian caucasians 2004 0,69 Canada Yes Morgan Model USA System; Med Science wedge spirometer model 570, PK Morgan Rolling seal spirometer, Stead wells spirometer Marsh, S. et al. Complete reference ranges for pulmonary function tests from a single population 2006 0,69 New Zealand Yes Jaegar Master Screen Body 266 (156/110) 18-70 12898 (6419/6479) 20-44 Roca, J. et al. References values for forced spirometry 1998 0,67 England No Sensor Medics 2130; Jaeger Pneumolab Vitalograph; Morgan Spirograph DS12; Sensor Medics Pne 12050; Spirotech S500; Hewlett Packard Falaschetti, E. et al. Prediction equations for normal and low lung function from the Health Survey for 2004 0,6 England Yes Vitalograph Escort Spirometer 3053 (556/2497) 16-75 Muhammad Asif Memon et al. Spirometric reference values in healthy, nonsmoking, urban Pakistani population Yes Micromedical, Microloop and Microrint (airway resistant) in Conjunction with spida 5 PC software 504 (183/321) 15-65 2007 0,6 Pakistan Results ATS/ERS criteria Author Conclusion 20 18 Years Years Old Old A. Langhammer et al. 0,75 Smolej, N. et al. 0,93 M. Susan Marion et al. 0,88 45 Years Old 65 Years Old 74 Years Old 80 Years Old 86 Years Old * The best scored article (93%) refers to a population with a very limited age * The second best scored article has a larger range of age but is still limited Discussion * The third best scored article, in spite of having a much lower score than the others has a larger age range * There is a limited number of studies of this type published each year * All but one study follow ATS/ERS criteria showing an effort for the standardization of this studies *There is the need for a study with a good sample size that covered a wide range of ages Discussion *We don’t have enough data to affirm that there are equations that can be applied in Portugal: a study should be conducted *The average score for methods (73%) and results (59%) were the lowest. Suggesting that most studies are frail in the most important topics of the check-list. Web site Plan Information Sources 1. Baur, X., S. Isringhausen-Bley, et al. (1999). "Comparison of lung-function reference values." International Archives of Occupational and Environmental Health 72(2): 69-83. 2. Enright P. Testing your lungs: spirometry [Internet]. European Lung Foundation. Available from: http://www.european-lung-foundation.org 3. Garcia-Rio, F., J. M. Pino, et al. (2004). "Spirometric reference equations for European females and males aged 65-85 yrs." Eur Respir J 24(3): 397-405. 4. Hughes, J.M.B.,Interpreting pulmonary function tests [Internet] ERS Education –Your best online source of CME in respiratory medicine [2007] European Respitatory Society. Available from: http://www.erseducation.org/media/2009/pdf/103639.pdf 5. Ip, M. S.-m., F. W.-s. Ko, et al. (2006). "Updated Spirometric Reference Values for Adult Chinese in Hong Kong and Implications on Clinical Utilization" Chest 129(2): 384-392. 6. Kuster, S. P., D. Kuster, et al. (2008). "Reference equations for lung function screening of healthy neversmoking adults aged 18-80 years." Eur Respir J 31(4): 860-868. 7. Liberati, A., D. G. Altman, et al. (2009). "The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration." Journal of Clinical Epidemiology 62(10): e1-e34. 8. Miller, Martin R. Interactive Course on Spirometry Training [Internet] ERS Education –Your best online source of CME in respiratory medicine [2007] European Respitatory Society. Available from: http://www.ers-education.org/pages/default.aspx?id=326 9. Unknown. STROBE Statement - checklist of items that should be included in reports of observational study [Internet] Strobe Statement: Strengthening the reporting of observational studies in epidemiology. [2009] ISPM - University of Bern. Available from: http://www.strobe-statement.org LEITE, Ana; PEIXOTO, Cláudia; MOURA, Diana; MARTINS, Diana; FERNANDES, Luís; ALMEIDA, Maria; BRITO, Nuno; ALMEIDA, Pedro; DIOGO, Pedro; MONTEIRO, Sara; PIMENTA, Sofia Class 1; turma1intromed@gmail.com 2009/2010