Introductory Practical DATA collection
advertisement
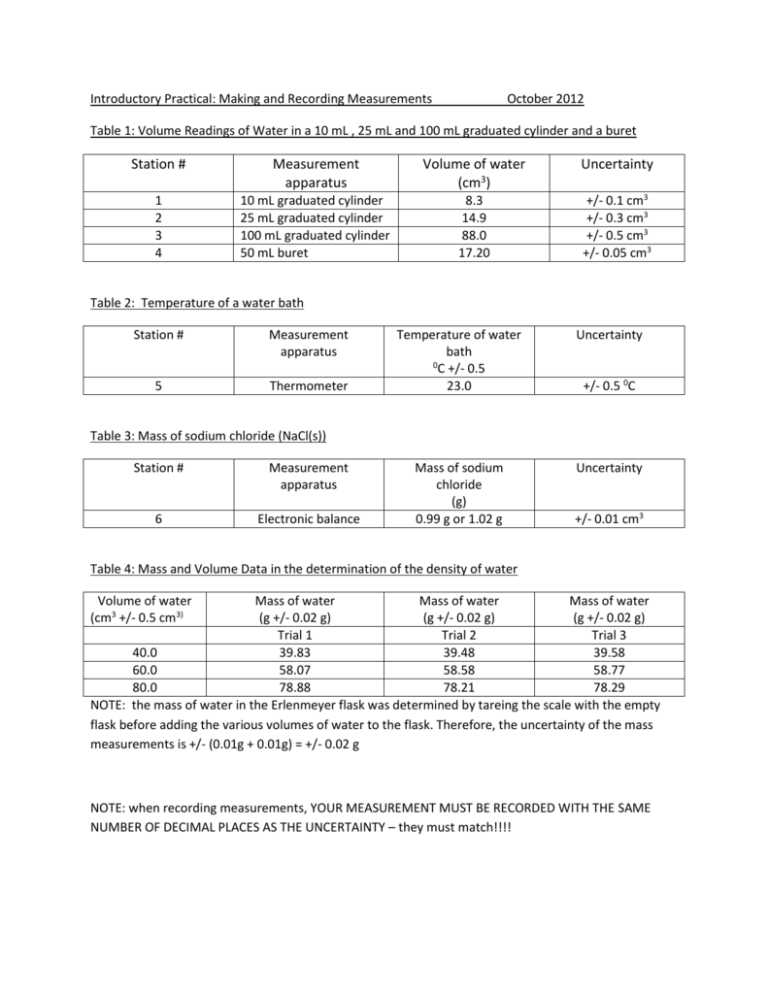
Introductory Practical: Making and Recording Measurements October 2012 Table 1: Volume Readings of Water in a 10 mL , 25 mL and 100 mL graduated cylinder and a buret Station # Measurement apparatus Volume of water (cm3) Uncertainty 1 2 3 4 10 mL graduated cylinder 25 mL graduated cylinder 100 mL graduated cylinder 50 mL buret 8.3 14.9 88.0 17.20 +/- 0.1 cm3 +/- 0.3 cm3 +/- 0.5 cm3 +/- 0.05 cm3 Table 2: Temperature of a water bath Station # Measurement apparatus 5 Thermometer Temperature of water bath 0 C +/- 0.5 23.0 Uncertainty Mass of sodium chloride (g) 0.99 g or 1.02 g Uncertainty +/- 0.5 0C Table 3: Mass of sodium chloride (NaCl(s)) Station # Measurement apparatus 6 Electronic balance +/- 0.01 cm3 Table 4: Mass and Volume Data in the determination of the density of water Volume of water (cm3 +/- 0.5 cm3) Mass of water Mass of water Mass of water (g +/- 0.02 g) (g +/- 0.02 g) (g +/- 0.02 g) Trial 1 Trial 2 Trial 3 40.0 39.83 39.48 39.58 60.0 58.07 58.58 58.77 80.0 78.88 78.21 78.29 NOTE: the mass of water in the Erlenmeyer flask was determined by tareing the scale with the empty flask before adding the various volumes of water to the flask. Therefore, the uncertainty of the mass measurements is +/- (0.01g + 0.01g) = +/- 0.02 g NOTE: when recording measurements, YOUR MEASUREMENT MUST BE RECORDED WITH THE SAME NUMBER OF DECIMAL PLACES AS THE UNCERTAINTY – they must match!!!!







