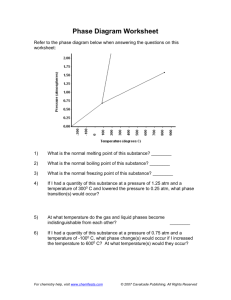
Worksheet C: Triple Point Name What is the relationship between
advertisement
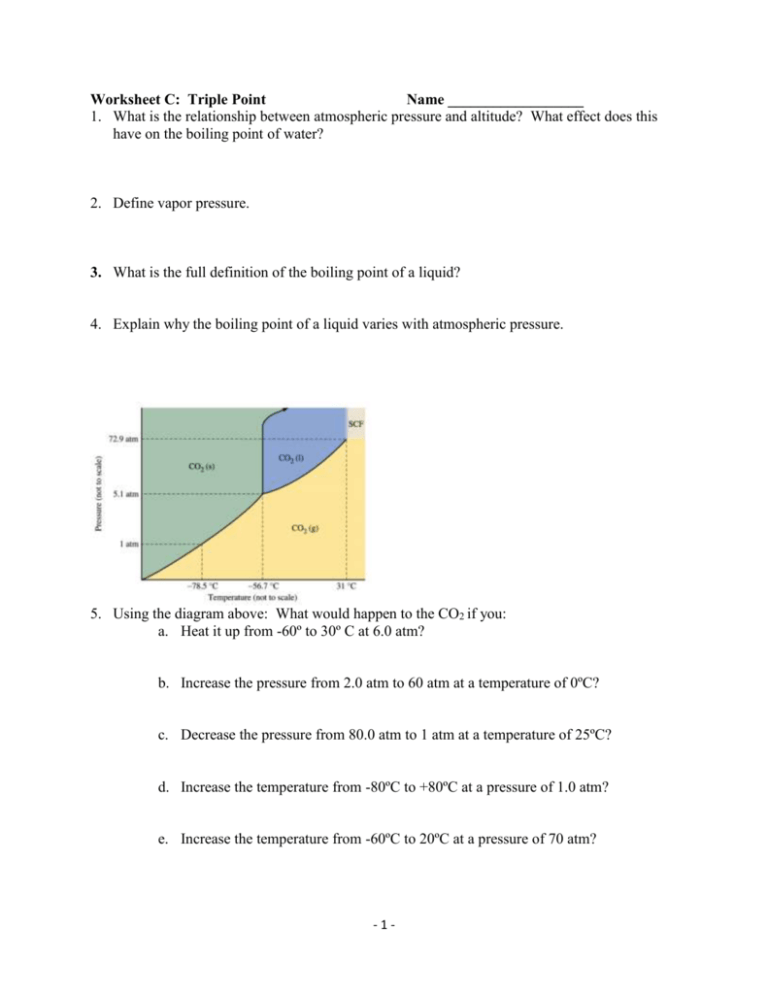
Worksheet C: Triple Point Name __________________ 1. What is the relationship between atmospheric pressure and altitude? What effect does this have on the boiling point of water? 2. Define vapor pressure. 3. What is the full definition of the boiling point of a liquid? 4. Explain why the boiling point of a liquid varies with atmospheric pressure. 5. Using the diagram above: What would happen to the CO2 if you: a. Heat it up from -60º to 30º C at 6.0 atm? b. Increase the pressure from 2.0 atm to 60 atm at a temperature of 0ºC? c. Decrease the pressure from 80.0 atm to 1 atm at a temperature of 25ºC? d. Increase the temperature from -80ºC to +80ºC at a pressure of 1.0 atm? e. Increase the temperature from -60ºC to 20ºC at a pressure of 70 atm? -1- Worksheet C: Triple Point Name __________________ 1. What is the relationship between atmospheric pressure and altitude? What effect does this have on the boiling point of water? The higher the altitude, the lower the atmospheric pressure. This makes it easier for liquid molecules to go into the vapor state thus lowering the boiling point 2. Define vapor pressure. the pressure of the vapor over a liquid at equilibrium (meaning that as many molecules are going into the vapor state as there are gas molecules going back into the liquid state) this is measured in a closed container. 3. What is the full definition of the boiling point of a liquid? When vapor pressure = atmospheric pressure 4. Explain why the boiling point of a liquid varies with atmospheric pressure. Applying the definition of boiling point it when atmospheric pressure changes so would vapor pressure which would affect boiling point 5. Using the diagram above: What would happen to the CO2 if you: a. Heat it up from -60º to 30º C at 6.0 atm? Melting would occur b. Increase the pressure from 2.0 atm to 60 atm at a temperature of 0ºC? Condensation would occur c. Decrease the pressure from 80.0 atm to 1 atm at a temperature of 25ºC? Vaporization would occur d. Increase the temperature from -80ºC to +80ºC at a pressure of 1.0 atm? Nothing, it would remain in the gas state e. Increase the temperature from -60ºC to 20ºC at a pressure of 70 atm? Melting would occur -2-