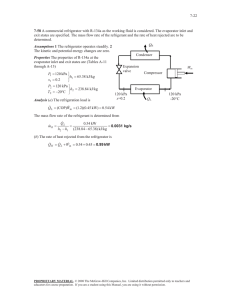
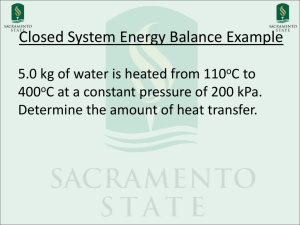
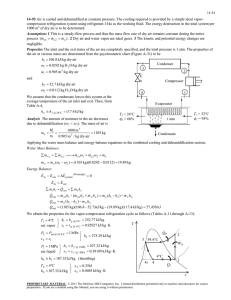
01-27-10
advertisement

State and Equilibrium State Postulate: State of a simple, compressible system is completely defined by two independent, intensive properties Keywords: State, simple compressible, independent, intensive Process and Cycles Quasi-equilibrium Isothermal, isobaric, adiabatic Forms of Energy Microscopic or macroscopic Internal energy – sensible, latent, chemical, nuke Kinetic and potential energies Heat and Work Heat – temperature difference (3 forms) Work – anything else (shaft, electricity, boundary, spring, etc.) Sign conventions, path vs. point functions Characteristics of heat and work Energy Efficiency Desired output / required input Heating value of a fuel Propagating efficiencies Example: Water Heater Gas: 55% Electric: 90% Why would gas be better? Example Your boss is inspired to “do something” about global warming. He decides to replace all of the 100 W lightbulbs in your company’s building (800 total) with compact florescent bulbs (19 W). The cfl’s cost $2.00 each, plus $0.75 labor to install. Electricity costs your company $0.09 / kW-hr and the bulbs are on for an average of 12 hours per day, five days per week. The bulbs are expected to last 5 years each. Does the energy savings justify the added cost of the bulbs? Phase Change Processes Lets boil some water… Property Tables See handout… Given Determine 80oC, 200 kPa v 225oC, 100 kPa h 150oC, X = 0.57, 2 kg P, V 500 kPa, 0.200 m3/kg u The evaporator of a refrigerator operates with R-134a at a pressure of 120 kPa. Assuming that the fluid in the evaporator is a saturated mixture, what is the temperature in the evaporator? One kg of R134a fills a 0.14 m3 weighted piston cylinder device at a temperature of 26.4oC. The container is heated until the temperature is 100oC. Determine the final volume of the R-134a. A piston-cylinder device initially contains 1.4 kg saturated liquid water at 200oC. Now heat is transferred to the water until the volume quadruples and the cylinder contains saturated vapor only. Determine (a) the initial volume of the tank (b) the final temperature and pressure (c) the internal energy change of the water Determine the specific volume of superheated water vapor at 10 MPa and 400oC, using (a) the ideal gas equation (b) the generalized compressibility chart (c) the steam tables A person boils water in a 30 cm diameter pot that is covered with a well fitting lid. The rising water vapor displaces all of the air in the pot. Then, it is removed from the heat and allowed to cool to the room temperature of 20oC. The total mass of water and pot is 4 kg. Now, the person tries to open the pot by lifting the lid up. Assuming that no air has leaked into the pot during cooling, determine if the lid will open or if the pot will move up with the lid.