Lecture 12-13: Planetary atmospheres
advertisement

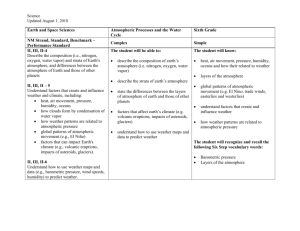
Lecture 12-13: Planetary atmospheres
o
Topics to be covered:
o Atmosphere composition.
o Atmospheric pressure.
o Atmospheric temperature.
o Atmospheric retention.
PY4022
Primary atmosphere
o
A planet’s primary atmosphere comes from nebular material in accretion disk.
o Mainly H, H2 and He.
o Trace elements also present in CO2, CH4, N2, H2O, NH3.
o
If planet’s gravity isn’t strong enough or surface temperature is too large, these
elements escape, leaving planet without an atmosphere.
o
Solar wind can also drag material from the atmosphere.
o Relevant for planets without significant magnetospheres (e.g., Mars).
o
For the terrestrial planets, most of the H escaped, leaving heavier gases such as
argon, neon and ammonia concentrated near the surface.
PY4022
Secondary atmosphere
o
Rocks and planetesimals which combined to
form each planet had trapped gasses.
o During formation, gases released from interior.
o Differentiation caused them to rise to the
outer surface of the planet.
o Released via volcanism.
o Comets/meteors containing water and gas
collided with the planets (H2O, CH4, CO2).
Mount Etna - March 2005
o
Volcanic gasses account for most of Earth's
atmosphere. Primitive atmosphere contained H2,
H2O, CO and H2S.
o
Biological activity: photosynthesis converts CO2
to O2.
(credit Reuters/Irish Times)
PY4022
Atmospheric pressure
o
Assume hydrostatic equilibrium:
International Civil Aviation Organisation
(ICAO) Standard Atmosphere
dP
g
dh
o As = P/RT and setting H = RT/ g =>
h 1
P P0 exp
dh
0 H
where P0 is pressure at surface and H is scale
height.
o
For Earth, H ~ 8 km.
o
Scale height implies planets with low gravity or
high temperature will have extended atmosphere.
o
Can also write:
0 exp 0
h
1
dh
H
PY4022
Atmospheric temperature
o
Atmosphere not isothermal.
function of height.
Structured
as
o
Troposphere: Lowest region in atmosphere. On
Earth, goes from ground to ~17 km. Weather and
clouds form from trace elements of condensable
gases. Temperature generally decreases with
altitude.
o
Stratosphere: T increases with altitude due to
absorption of UV. Extends to ~50 km (on Earth).
No clouds.
o
Mesosphere: On Earth T quickly decrease with
height
o
Thermosphere: T increases with altitude due to
strong UV flux. Includes the exosphere and part
of the ionosphere. On Earth, T~1000K at 500
km.
PY4022
Atmospheric equilibrium temperature
Pabs
Lsun
2
R
W
4d 2
o
At distance d from Sun, a planet of radius R receives:
where Lsun is the solar luminosity in W.
o
At 1 AU, Flux (F) = Lsun/ 4 d2 = 3.85 x 1026/
4 (1.49 x 1011)2 = 1370 W/m2.
o
But, a fraction (A) of power is reflected - A called planetary albedo (0 A 1).
o A = 1: Total reflection.
o A = 0: Total absorption.
o
o
Fraction (1 - A) is absorbed by surface of planet.
Rocks are poor reflectors and have low albedos,
ice is a moderate reflector.
Planet
A
Earth
0.37
Moon
0.12
Venus
0.65
Jupiter
0.52
Pluto
0.3
PY4022
Atmospheric equilibrium temperature
o
So, a planet of radius R and distance d from Sun will absorb:
Pabs Fsun R 2 (1 A) W
Eqn. 1
where Fsun=1370/d2(AU). Assuming planet is blackbody will radiate energy back
into space at a rate give by the Stefan-Boltzmann Law
Pemitt = 4 R2 e T4
W
Eqn. 2
where e is emissivity. Accounts for fact that planets not perfect blackbodies.
1370(1 A)
T
2
4e d
1/ 4
o
In equilibrium, Eqn. 1 = 2. Rearranging gives,
o
Temperature of planet is not related to how massive or its surface area.
PY4022
Atmospheric equilibrium temperature
o
Substituting for constants,
(1 A)
T 278
2
e d(AU)
1
0
0
0
1/ 4
A
=
0
V
e
n
u
s
(
r
u
n
a
w
a
y
"
g
r
e
e
n
h
o
u
s
e
e
f
f
e
c
t
"
)
A
=
0
.
9
o
E.g., for Earth, T = 248 K and for Moon, T
= 269 K
M
o
o
n
E
a
r
t
h
M
a
r
s
P
e
r
f
e
c
tb
la
c
k
b
o
d
y
Observed temperatures are: Earth T = 288
K and Moon T = 252 K
M
e
r
c
u
r
y
1
0
0
Temperature(K)
o
J
u
p
it
e
r
S
a
t
u
r
n
s
lo
w
r
o
t
a
t
io
n
+
n
o
a
t
m
o
s
p
h
e
r
e
U
r
a
n
u
s
N
e
p
t
u
n
e
P
lu
t
o
o
Earth is not a perfect blackbody:
o Some solar heat is conducted into
surface rock and oceans - this is a
form of ‘stored’ heat energy
o Earth has atmosphere which acts like
thermal blanket, ‘trapping’ infrared
radiation.
21
/
4
T
2
7
8
{(
1
-A
)/e
d
(
a
u
)}
p
l
a
n
e
t=
A
=
a
lb
e
d
o
,e
=
e
m
is
s
iv
it
y
=
1
1
0
0
.
1
1
1
0
D
i
s
t
a
n
c
e
(
A
U
)
PY4022
Greenhouse effect
o
When sunlight reaches Earth, much
passes to surface, because atmosphere is
transparent to visible/very near-infrared.
o
Ground absorbs V-NIR, and heats up.
o
Then re-radiates energy. T ground lower
than Sun’s surface, so radiation emitted at
longer wavelengths (Wien’s Law) in the
mid-IR (MIR).
o
Atmosphere was transparent to V-NIR
light, is opaque to the MIR. On Earth,
H2O and CO2 absorb strongly in MIR.
o
Energy trapped near surface. Eventually
equilibrium is achieved, but at a higher T.
PY4022
Greenhouse effect
QuickTime™ and a
decompressor
are needed to see this picture.
PY4022
Runaway Greenhouse effect
o
Greenhouse effect is much more
prominent on Venus.
o
Venus has thick atmosphere of 96%
CO2, 3.5% N2 and 0.5% other gases.
o
Venus originally cooler and had
greater abundance of water several
billion years ago. Also, most of its
carbon dioxide was locked up in the
rocks.
o Because Venus was closer to Sun than Earth, water never liquified and remained in
the atmosphere to start the greenhouse heating. As Venus heated up, CO2 in the
rocks was “baked out”. Increase of atmospheric CO2 enhanced greenhouse heating
and baked more carbon dioxide => runaway feedback loop.
PY4022
Atmospheric retention
o
Energy of a molecule in atmosphere can be written:
GMm
0
r
o A particle will escape from planet if has enough KE. Escape speed v = vesc, needed
to escape from r = R is therefore:
E total E k E p 1/2mv 2
o
o
v esc
2GM
R
2
From kinetic theory, 1/2mvtherm 3/2kT therefore,
3kT
v therm
m
Lightest particles (H and He) have highest speeds and escape preferentially if T is
large enough for particles to have vtherm > vesc.
PY4022
Atmospheric retention
o
A planet will retain its atmosphere if vtherm vesc
Escape
o
The escape condition occurs when
Exosphere
3kT
2GM
m
R
2GMm
Tesc
3kR
o
Atmosphere
Random collisions
Ground
The region where this condition is met is called the exosphere.
o
If surface temperature is large, planet will loose atmosphere. Also, small planets
find it difficult to hold onto atmospheres.
o
For a given planet or satellite of mass M and radius R the atmospheric retention
condition is
Tatm < Tesc
PY4022
Atmospheric retention
2GM
3kT
3kTR
m
R
m
2GM
o
For a given molecule to be retained:
o
Definition: m = mH
o where is molecular weight and mH is mass of H-atom (mH = 1.67 x 10-27 kg).
o so, for hydrogen = 1,
and for helium = 4
o hence at a given temperature the He atoms will be moving slower than H atoms
o
For Earth
o Tatm = 288 K and vesc = 11.2 km s-1
o Hence, escape for all molecules with 4
o So, don’t expect to find much H or He.
o
For Jupiter
o Tatm = 134 K and vesc = 59.5 km s-1
o Hence, escape for all molecules with < 0.06
o So, nothing escapes, since hydrogen with = 1 is the ‘lightest’ gas element.
Observations show that Jupiter is a H and He gas giant.
PY4022
Atmospheric retention
o
o
As vtherm ~ m-1/2 and ~T1/2, light gases have higher
speeds and hot gases have higher speeds.
Gas giants are massive planets with high escape
speeds and cold temperatures, so light gases such as
H and He retained. Small rocky bodies are closer to
the Sun, have higher temperatures and less mass, and
so lack H and He - some have no atmosphere.
o
Even if vtherm < vesc, some particles will escape due to
the ‘high-speed’ tail of the Maxwellian distribution.
o
For a planet to ‘hold’ an atmosphere over the age of
the Solar System (~4.5 billion years), the escape
condition is more like vesc > 10 vtherm
o
The factor of 10 accounts for the high-velocity tail of
the Maxwellian distribution of speeds.
Oxygen
Helium
Hydrogen
PY4022
Atmospheric retention
R
e
t
e
n
t
i
o
n
o
fA
t
m
o
s
p
h
e
r
i
c
G
a
s
e
s
1
0
0
o Escape velocity:
v esc
2GM
R
J
u
p
it
e
r
H
y
d
r
o
g
e
n
N
e
p
t
u
n
e
S
a
t
u
r
n
H
e
liu
m
U
r
a
n
u
s
E
a
r
t
h
o
V
e
n
u
s
1
0
3kT
Thermal velocity: v
therm
m
N
2
M
a
r
s
C
O
2
M
e
r
c
u
r
y
T
it
a
n
X
e
Velocity(km/s)
T
r
it
o
n
o Consequences:
H
O
2
M
o
o
n
o Light
elements escape more easily.
o Hot planets “burn off” their atmosphere.
o Small planets cannot hold onto atmosphere.
1
P
lu
t
o
C
e
r
e
s
V
e
s
t
a
P
a
lla
s
N
B
:lin
e
s
s
h
o
w
t
e
n
t
im
e
s
m
e
a
n
m
o
le
c
u
la
rs
p
e
e
d
s
P
la
n
e
t
s
G
a
lile
a
n
m
o
o
n
s
T
r
it
o
n
a
n
d
T
it
a
n
M
in
o
rP
la
n
e
t
s
0
.
1
1
0
0
1
0
0
0
T
e
m
p
e
r
a
t
u
r
e
(
K
)
PY4022
QuickTime™ and a
TIFF (Uncompressed)
decompressor
Venus
Express
are needed to see this picture.
o
What is the mechanism and driving force of
the super-rotation of the atmosphere?
o
What are the basic processes in the general
circulation of the atmosphere?
o
What is composition and chemistry of lower
atmosphere and clouds?
o
What is the past and present water balance in
the atmosphere?
o
What is the role of the radiative balance and
greenhouse effect?
o
Is there currently volcanic and/or tectonic
activity on the planet
QuickTime™ and a
TIFF (Uncompressed) decompressor
are needed to see this picture.
o Arrived at Venus in April 2006.
PY4022