Week 13 Making Chemical Compounds
advertisement

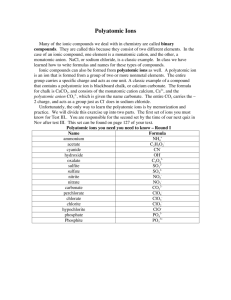
Making Chemical Compounds 7.1 Chemical Names and Formulas Review • Ionic charges (groups of the periodic table) • What makes up ionic compounds? • What makes up molecular (or covalent) compounds? • Cation? • Anion? • Molecule? • Chemical formula? Making Ionic Compounds • Ionic compounds are made up of a _______ and a _______. • When making an ionic compound, the _______ is written first, then the ________. • Write the ionic charge in the top right-hand corner of the symbol. • Criss-cross the charges (move the number of the charge from the top right-hand corner to the opposite bottom corner). Examples 1. 2. 3. 4. 5. 6. 7. Sodium and chlorine Sodium and oxygen Sodium and nitrogen Magnesium and sulfur Magnesium and phosphorus Aluminum and bromine Aluminum and oxygen Naming Ionic Compounds 1. The name of the cation goes first 2. Drop the suffix (last syllable) of the anion, and add –ide 1. For example, chlorine = chloride, sulfur = sulfide Examples • • NaCl = Sodium chloride CaO = Calcium oxide ▫ Practice individually on page 223, #2 Transition Metals • Metals in groups 3-12 have more than one ionic charge because of their mobile valence electrons (remember the sea of electrons?) • When naming these compounds, we will use the Stock system. • Put the number of the charge on the transition metal as a roman numeral in parentheses. ▫ For example: Cu2+ = Copper (II), Sn4+ = Tin (IV) • Practice individually on page 225, #1 and 2 Polyatomic Ions • You must memorize the names and formulas of the following polyatomic ions (p.226): ▫ ▫ ▫ ▫ Acetate Cyanide Hydroxide Permanganate ▫ The rest are provided on the back of your periodic table Compounds with Polyatomic Ions • Polyatomic ions act as one unit when forming compounds. • They have one ionic charge (equal to the sum of all of the individual atoms’ ionic charges) • They will act as either a cation (NH4+) or an oxyanion (ions with oxygen) Examples • • • • Sodium and hydroxide Sodium and sulfate Sodium and phosphate Ammonium and nitrate • Practice individually on page 227, #1 Naming Compounds with Polyatomic Ions • Polyatomic ions keep their name unaltered in compounds ▫ This is because the suffix of a polyatomic ion indicates the number of oxygens in the ion. Ex. Sulfate is (SO42-), but sulfite is (SO32-) ▫ In compounds with ammonium, single-atom anions (like chlorine, sulfur, etc.) will change to the –ide ending. Ex. Ammonium chloride is NH4Cl. • Practice individually on page 227, #2 Making Molecular Compounds • Molecular compounds do NOT criss-cross their charges. • Molecules are named using numerical prefixes (p.228). ▫ Ex. Carbon dioxide is CO2, because di- means two. • Practice together on page 229, #1 and 2 Prefixes: 1= mono2 = di3 = tri4 = tetra5 = penta6 = hexa7 = hepta8 = octa9 = nona10 = deca- Naming Acids • Binary acids (H-anion) include the name of the anion with hydro–ic acid. ▫ Ex. Hydrochloric acid is HCl ▫ What would Hydrosulfuric acid be? • Oxyacids (H-oxyanion) include the name of the polyatomic ion with the suffix –ic acid. ▫ Ex. Sulfuric acid is H2SO4 ▫ What would nitric acid be? You need to know: • From the chart on page 230, you need to know the following: ▫ ▫ ▫ ▫ ▫ ▫ ▫ ▫ hydrofluoric acid hydrochloric acid hydrobromic acid phosphoric acid nitric acid sulfuric acid acetic acid carbonic acid. Homework • Homework: Complete 7.1 section review NOW