Enrichment - Rate Equations to Determine Reaction Order
advertisement

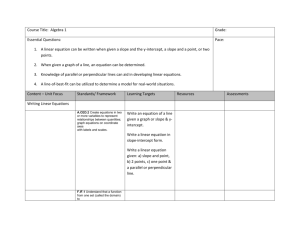
Enrichment - Rate Equations to Determine Reaction Order • The equation for a straight line is: y mx b • Compare this equation to the rearranged first order rate-law. Enrichment - Rate Equations to Determine Reaction Order y mx b ln A a k t ln A0 • Now we can interpret the parts of the equation as follows: – y can be identified with ln[A] and plotted on the y-axis. – m can be identified with –ak and is the slope of the line. – x can be identified with t and plotted on the x-axis. Enrichment - Rate Equations to Determine Reaction Order • Example 16-9: Concentration-versus-time data for the thermal decomposition of ethyl bromide are given in the table below. Use the following graphs of the data to determine the rate of the reaction and the value of the rate constant. C2 H5Brg C2H4g HBrg at 700K Enrichment - Rate Equations to Determine Reaction Order Time (min) [C2H5Br] 0 1.00 1 0.82 2 0.67 3 0.55 4 0.45 5 0.37 ln [C2H5Br] 0.00 -0.20 -0.40 -0.60 -0.80 -0.99 1/[C2H5Br] 1.0 1.2 1.5 1.8 2.2 2.7 Enrichment - Rate Equations to Determine Reaction Order • We will make three different graphs of the data. 1 Plot the [C2H5Br] (y-axis) vs. time (x-axis) – If the plot is linear then the reaction is zero order with respect to [C2H5Br]. 2 Plot the ln [C2H5Br] (y-axis) vs. time (xaxis) – If the plot is linear then the reaction is first order with respect to [C2H5Br]. 3 Plot 1/ [C2H5Br] (y-axis) vs. time (x-axis) – If the plot is linear then the reaction is second Enrichment - Rate Equations to Determine Reaction Order • Plot of [C2H5Br] versus time. – Is it linear or not? [C2H 5Br] [C2H5Br] vs. time 1.2 1 0.8 0.6 0.4 0.2 0 0 1 2 3 Time (min) 4 5 Enrichment - Rate Equations to Determine Reaction Order • Plot of ln [C2H5Br] versus time. – Is it linear or not? ln [C2H5Br] vs. time 0 ln [C 2H5Br] -0.2 0 1 2 3 -0.4 -0.6 -0.8 -1 -1.2 Time (min) 4 5 Enrichment - Rate Equations to Determine Reaction Order • Plot of 1/[C2H5Br] versus time. – Is it linear or not? 1/[C2H5Br] vs. time 1/[C 2H5Br] 3 2 1 0 0 1 2 3 Time (min) 4 5 Enrichment - Rate Equations to Determine Reaction Order • Note that the only graph which is linear is the plot of ln[C2H5Br] vs. time. – Thus this is a first order reaction with respect to [C2H5Br]. • Next, we will determine the value of the rate constant from the slope of the line on the graph of ln[C2H5Br] vs. time. – Remember slope = y2-y1/x2-x1. y 2 - y1 0.80 (0.20) slope x 2 - x1 4 1 min 0.60 slope 0.20 min -1 3 min Enrichment - Rate Equations to Determine Reaction Order • From the equation for a first order reaction we know that the slope = -a k. – In this reaction a = 1. slope -0.20 -k Thus the rate constant k 0.20 min . -1 Enrichment - Rate Equations to Determine Reaction Order • The integrated rate equation for a reaction that is second order in reactant A and second order overall. 1 1 akt A A0 • This equation can be rearranged to: 1 1 akt A A0 Enrichment - Rate Equations to Determine Reaction Order • Compare the equation for a straight line and the second order rate-law expression. y mx b 1 1 akt A A0 • Now we can interpret the parts of the equation as follows: – y can be identified with 1/[A] and plotted on the y-axis. – m can be identified with a k and is the slope of the line. – x can be identified with t and plotted on the x-axis – b can be identified with 1/[A]0 and is the y-intercept. Enrichment - Rate Equations to Determine Reaction Order • Example 16-10: Concentration-versustime data for the decomposition of nitrogen dioxide are given in the table below. Use the graphs to determine the rate of the reaction and the value of the rate constant 2 NO2 g 2 NOg O2g at 500K Enrichment - Rate Equations to Determine Reaction Order Time (min) [NO2] 0 1.0 1 0.53 2 0.36 3 0.27 4 0.22 5 0.18 ln [NO2] 0.0 -0.63 -1.0 -1.3 -1.5 -1.7 1/[NO2] 1.0 1.9 2.8 3.7 4.6 5.5 Enrichment - Rate Equations to Determine Reaction Order • Once again, we will make three different graphs of the data. 1. Plot [NO2] (y-axis) vs. time (x-axis). – If the plot is linear then the reaction is zero order with respect to NO2. 2. 3. Plot ln [NO2] (y-axis) vs. time (x-axis). • If the plot is linear then the reaction is first order with respect to NO2. Plot 1/ [NO2] (y-axis) vs. time (x-axis). – If the plot is linear then the reaction is second order with respect to NO2. Enrichment - Rate Equations to Determine Reaction Order • Plot of [NO2] versus time. – Is it linear or not? [NO2] [NO2] vs. time 1.2 1 0.8 0.6 0.4 0.2 0 0 1 2 3 Time (min) 4 5 Enrichment -Rate Equations to Determine Reaction Order • Plot of ln [NO2] versus time. – Is it linear or not? ln [NO2] vs. time 0 0 1 2 3 ln [NO2] -0.5 -1 -1.5 -2 Time (min) 4 5 Enrichment - Rate Equations to Determine Reaction Order • Plot of 1/[NO2] versus time. – Is it linear or not? 1/[NO2] 1/[NO2] vs.time 6 5 4 3 2 1 0 0 1 2 3 Time (min) 4 5 Enrichment - Rate Equations to Determine Reaction Order • Note that the only graph which is linear is the plot of 1/[NO2] vs. time. • Thus this is a second order reaction with respect to [NO2]. • Next, we will determine the value of the rate constant from the slope of the line on the graph of 1/[NO2] vs. time. Enrichment - Rate Equations to Determine Reaction Order y 2 - y1 5.50 (1.90) 1 M slope x 2 - x1 5 1 min 3.60 1 M slope 0.90 1 M min 4 min • From the equation for a first order reaction we know that the slope = a k – In this reaction a = 2. slope 0.90 2 k Thus the rate constant k 0.45 M 1 min -1