PPT - American Academy of Pediatrics
advertisement

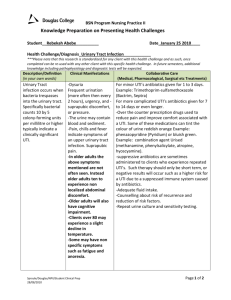
TM TM Prepared for your next patient. AAP Guideline for the Diagnosis and Management of UTIs in Febrile Infants Unanswered Questions and Unquestioned Answers Kenneth B. Roberts, MD, FAAP Professor of Pediatrics (Emeritus) University of North Carolina TM Disclaimers Statements and opinions expressed are those of the authors and not necessarily those of the American Academy of Pediatrics. Mead Johnson sponsors programs such as this to give healthcare professionals access to scientific and educational information provided by experts. The presenter has complete and independent control over the planning and content of the presentation, and is not receiving any compensation from Mead Johnson for this presentation. The presenter’s comments and opinions are not necessarily those of Mead Johnson. In the event that the presentation contains statements about uses of drugs that are not within the drugs' approved indications, Mead Johnson does not promote the use of any drug for indications outside the FDA-approved product label. TM AAP 2011 Clinical Practice Guideline Diagnosis and Management of the Initial UTI in Febrile Infants and Children, 2 to 24 Months* *Guideline: Pediatrics. 2011;128(3):595–610 Technical report: Pediatrics. 2011;128(3):e749–e770 TM Revision of 1999 Guideline • Routine for American Academy of Pediatrics (AAP) to revise guidelines • New evidence since 1999 • New explicit reporting format – “Recommendations” now “Action Statements” – Aggregate evidence quality • • • • • • • Benefits Harms/risks/costs Benefit-harms assessment Value judgments Role of patient preferences Exclusions Intentional vagueness – Policy level (strength of recommendation) TM Evidence Quality A. Well-designed RCTs or diagnostic studies on relevant population Preponderance of Benefit or Harm Balance of Benefit and Harm Strong Recommendation B. RCTs or diagnostic studies with minor limitations; overwhelmingly consistent evidence from observational studies C. Observational studies (case-control and cohort design) D. Expert opinion, case reports, reasoning from first principles Abbreviation: RCTs, randomized controlled trials. Option Recommendation Option No Recommendation TM Evidence Quality X. Exceptional situations where validating studies cannot be performed and there is a clear preponderance of benefit or harm Preponderance of Benefit or Harm Strong Recommendation Recommendation TM AAP Subcommittee on Urinary Tract Infection (UTI) • • • • • • • • Stephen M. Downs, MD, MS: Epidemiology/informatics S. Maria E. Finnell, MD, MS: Epidemiology/informatics Stanley Hellerstein, MD: Pediatric nephrology Kenneth B. Roberts, MD, Chair: General pediatrics Linda D. Shortliffe, MD: Pediatric urology Ellen R. Wald, MD: Pediatric infectious diseases J. Michael Zerin, MD: Pediatric radiology Caryn Davidson, MA: AAP staff TM Driving Force from the 1960s TM Used with permission, ScienceCartoonsPlus.com TM What’s New in This Revision 1. Diagnosis – Abnormal urinalysis as well as positive culture – Positive culture = ≥50,000 colony-forming units (cfu)/mL – Assessment of likelihood of UTI 2. Treatment: Oral as effective as parenteral 3. Imaging: Voiding cystourethrography (VCUG) not recommended routinely after first febrile UTI 4. Follow-up: Emphasis on urine testing with subsequent febrile illnesses TM Population Addressed • Infants and young children, 2–24 months of age, with unexplained fever – Rate of UTI: ~5% – Rate of scarring: Higher than in older children TM Population Addressed • Infants and young children, 2–24 months of age, with unexplained fever – Rate of UTI: ~5% – Rate of scarring: Higher than in older children • Excludes: <2 months of age • Excludes: >24 months of age TM Content • Action Statements: 7 – Diagnosis: 3 – Treatment: 1 – Imaging: 2 – Follow-up: 1 • Areas for Research: 8 TM Action Statement 1 If a clinician decides that a febrile infant with no apparent source for the fever requires antimicrobial therapy because of ill appearance or another pressing reason, a urine specimen should be obtained by catheterization for both culture and urinalysis before an antimicrobial is given. Evidence quality: A Strong recommendation TM Methods of Collecting Specimen • Suprapubic aspiration: “Gold standard,” but – Variable success rates: 23–90% (higher with ultrasound guidance) – Requires technical expertise and experience – Often viewed as invasive – More painful than catheterization – May be no alternative in boys with severe phimosis or girls with tight labial adhesions TM Methods of Collecting Specimen • Bag urine – Can’t avoid getting “vaginal wash” in girl or contamination in uncircumcised boy. – Not suitable for culture. Negative culture rules out UTI, but Positive culture likely to be false-positive o 88% false-positive overall o 95% in boys o 99% in circumcised boys – Positive culture requires confirmation, which is not possible once antibiotic is started. TM Methods of Collecting Specimen • Catheterization – Compared to suprapubic aspiration: Sensitivity = 95% Specificity = 99% – Requires some skill, particularly in small infant girls. (Tip: If unsuccessful, leave catheter in.) TM Action Statement 2 If a febrile infant is assessed as not requiring immediate antimicrobial therapy, then the likelihood of UTI should be assessed. • If likelihood is low (<1%, <2%), it is reasonable to follow the child clinically. • If the likelihood is not low, there are two options: – Obtain specimen by catheter for culture and urinary analysis (UA). – Obtain specimen by any means for UA and only culture those with positive UA. TM Probability of UTI: Infant GIRLS Individual Factors • • • • • Race: White Age: <12 months Temperature: ≥39⁰C Fever: ≥2 days Absence of another source of infection Probability of UTI # of Factors Present ≤1% No more than 1 ≤2% No more than 2 TM Probability of UTI: Infant BOYS Individual Factors • • • • Race: Nonblack Temperature: ≥39⁰C Fever: >24 hours Absence of another source of infection # of Factors Present Probability of UTI ≤1% ≤2% Circumcised No Yes * No more than 2 None No more than 3 *Probability of UTI exceeds 1% even with no risk factors other than being uncircumcised. TM Action Statement 3 Diagnosis of UTI requires both: • Positive culture – ≥50,000 cfu/mL of uropathogen cultured from catheter specimen, AND • Positive urinalysis Evidence quality: C Recommendation TM Where Did 100,000 Come From? 90% Asymptomatic women in medical OPD Asymptomatic women with diabetes Asymptomatic women with cystocele Pts with diagnosis of pyelonephritis 80% 70% 60% 50% 40% 30% 20% 10% 0% 0 100-1 101-2 102-3 103-4 104-5 105-6 >106 Kass E. Asymptomatic infections of the urinary tract. Trans Assoc Am Phys. 1956;69:56–64 TM Urinalysis • Positive urinalysis required for diagnosis – Positive culture with “negative” urinalysis • Contamination • Asymptomatic bacteriuria • Urinalysis not sensitive enough • Positive – Dipstick: +LE (leukocyte esterase) and/or +nitrite – Microscopy: White blood cells ± bacteria TM Action Statement 4 Choice of route: Initiating treatment orally or parenterally is equally efficacious, so choice is based on practical considerations. Evidence quality: A Strong recommendation TM Action Statement 4 Choice of route: Initiating treatment orally or parenterally is equally efficacious, so choice is based on practical considerations. Evidence quality: A Strong recommendation Choice of drug: Based on local sensitivity patterns, adjusted according to sensitivity of particular uropathogen Evidence quality: A Strong recommendation TM Action Statement 4 Choice of route: Initiating treatment orally or parenterally is equally efficacious, so choice is based on practical considerations. Evidence quality: A Strong recommendation Choice of drug: Based on local sensitivity patterns, adjusted according to sensitivity of particular uropathogen Evidence quality: A Strong recommendation Duration of treatment: 7–14 days Evidence quality: B Recommendation TM Action Statement 5 Febrile infants with UTIs should undergo renal and bladder ultrasonography (RBUS), Evidence quality: C Recommendation TM Action Statement 5 Febrile infants with UTIs should undergo RBUS. Evidence quality: C Recommendation Why: • • Yield of abnormal findings: 12–16% Permanent renal damage (1 year later) – – • Sensitivity: 41% Specificity: 81% Actionable findings sufficient to warrant? TM Action Statement 5 Febrile infants with UTIs should undergo RBUS. Evidence quality: C Recommendation When: • Decide clinically: Within 48 hours if not responding to treatment as expected, unusually ill, or extenuating circumstances; otherwise, when convenient. TM Action Statement 6 VCUG is not recommended to be performed routinely after the first febrile UTI if RBUS is normal. Evidence quality: B Recommendation TM TM Action Statement 6 1. Garin EH, Olavarrio F, Garcia Nieto V, et al. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized controlled study. Pediatrics. 2006;117(3):626–632 2. Pennesi M, Travan L, Peratoner L, et al. Is antibiotic prophylaxis in childrfen with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized controlled trial. Pediatrics. 2008;121(6):e1489–e1494 3. Montini G, Rigon L, Zuccheta P, et al. Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics. 2008;122(5):1064–1071 4. Roussey-Kesler G, Gadjos V, Idres N, et al. Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low grade vesicoureteral reflux results from a prospective randomized study. J Urol. 2008;179(2):674–679 5. Craig JC, Simpson JM, Williams GJ, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748–1759 6. Brandström P, Esbjörner E, Herthelius M, et al. The Swedish reflux trial in children: III. Urinary tract infection pattern. J Urol. 2010;184(1):286–291 TM Action Statement 6 Reflux Grade N Prophylaxis No Prophylaxis # of Recurrences / Total N # of Recurrences / Total N P None 373 7 / 210 11 / 163 0.15 Grade I 72 2 / 37 2 / 35 1.00 Grade II 257 11 / 133 10 / 124 0.95 Grade III 285 31 / 140 40 / 145 0.29 Grade IV 104 16 / 55 21 / 49 0.14 1,091 TM Recurrence Rate of Febrile UTI By Reflux Grade, 1,091 Infants 2–24 Months 250 Prophylaxis NS No Prophylaxis 200 NS 150 NS 100 NS NS 50 0 None Grade I Grade II Grade III Grade of Vesico-Ureteral Reflux (VUR) Grade IV TM Recurrence Rate of Febrile UTI By Reflux Grade, 1,091 Infants 2–24 Months Recurrence 100% 80% Prophylaxis 60% No Prophylaxis NS 40% 20% NS NS NS NS None (N=373) Grade I (N=100) Grade II (N=257) 0% Grade of VUR Grade III (N=285) Grade IV (N=104) TM Action Statement 6 If RBUS is abnormal, VCUG may be part of additional imaging required to evaluate the abnormality. Evidence quality: B Recommendation Further evaluation should be conducted if there is a recurrence of febrile UTI. Evidence quality: X Recommendation TM Action Statement 6 After First UTI (N=100) 65 (65%) After Recurrence (N=10) 2.6 (26%) Grade I–III VUR 29 (29%) 5.6 (56%) Grade IV VUR Grade V VUR 5 (5%) 1 (1%) 1.2 (12%) 0.6 (6%) No VUR TM Action Statement 6 100% 80% 60% 40% 20% 0% 1 2 3 4 Risk of Renal Scarring by Number of UTIs Adapted from Jodul U. The natural history of bacteriuria in childhood. Infect Dis Clin North Am. 1987;1(4):713–729 5 TM Impact of a More Restrictive Approach to Urinary Tract Imaging After Febrile Urinary Tract Infection • N=103 • “By restricting urinary tract imaging after an initial febrile UTI [based on NICE guidelines, 2007], rates of voiding cystourethrography and prophylactic antibiotic use decreased substantially without increasing the risk of UTI recurrence within 6 months and without an apparent decrease in detection of high-grade VUR.” Schroeder AR, Abidari JM, Kirpekar R, et al. Impact of a more restrictive approach to urinary tract imaging after febrile urinary tract infection. Arch Pediatr Adolesc Med. 2011;165(11):1027–1032 TM Childhood Urinary Tract Infections as a Cause of Chronic Kidney Disease • N=1,576 • “VUR with UTI without structural abnormalities in the kidneys seems not to cause CKD.” • “Active treatment of VUR seems not to reduce the occurrence of CKD and, in large prospective followup studies, the renal function of patients with VUR has been well preserved.” Salo J, Ikäheimo R, Tapiainen T, et al. Childhood urinary tract infections as a cause of chronic kidney disease. Pediatrics. 2011;128(5):840–847 TM Action Statement 7 Following confirmation of UTI, parents or guardians should be instructed to seek prompt medical evaluation for future febrile illnesses to ensure that recurrent infections can be detected and treated promptly. Evidence quality: C Recommendation TM Areas for Research (8) 1. Relationship between UTIs and reduced renal function / hypertension 2. Alternatives to invasive collection of urine and culture 3. Role of VUR (and, thus, VCUG) 4. Role of prophylaxis (Randomized Intervention for Children with Vesicoureteral Reflux [RIVUR] study) 5. Genetics 6. Hispanics 7. Further treatment: What and for whom? 8. Duration of treatment TM Summary: What’s New . . . 1. Diagnosis – Abnormal urinalysis, as well as positive culture – Positive culture = ≥50,000 cfu/mL – Assessment of likelihood of UTI 2. Treatment: Oral as effective as parenteral 3. Imaging: VCUG not recommended routinely after first febrile UTI 4. Follow-up: Emphasis on urine testing with subsequent febrile illnesses TM For more information… On this topic and a host of other topics, visit www.pediatriccareonline.org. Pediatric Care Online is a convenient electronic resource for immediate expert help with virtually every pediatric clinical information need. Musthave resources are included in a comprehensive reference library and time-saving clinical tools. • Haven't activated your Pediatric Care Online trial subscription yet? It's quick and easy: simply follow the steps on the back of the card you received from your Mead Johnson representative. • Haven't received your free trial card? Contact your Mead Johnson representative or call 888/363-2362 today.