+ NO 2
advertisement
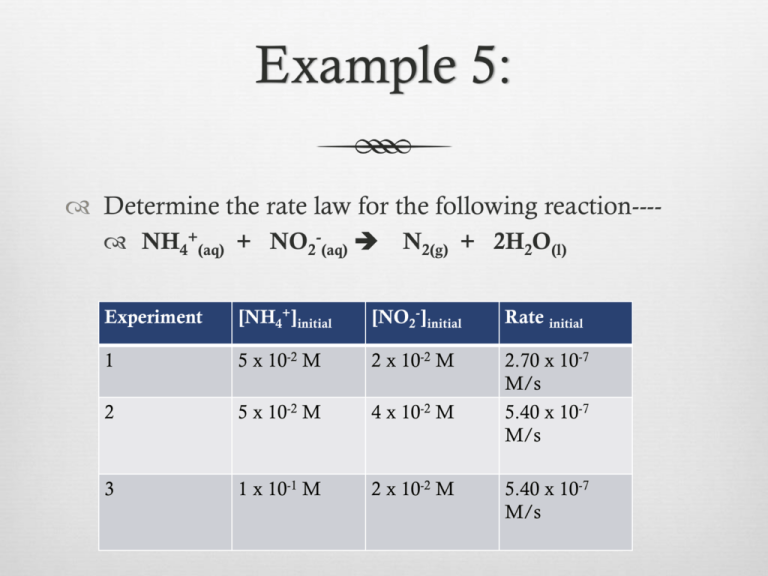
Example 5: Determine the rate law for the following reaction--- NH4+(aq) + NO2-(aq) N2(g) + 2H2O(l) Experiment [NH4+]initial [NO2-]initial Rate initial 1 5 x 10-2 M 2 x 10-2 M 2 5 x 10-2 M 4 x 10-2 M 2.70 x 10-7 M/s 5.40 x 10-7 M/s 3 1 x 10-1 M 2 x 10-2 M 5.40 x 10-7 M/s Zero-Order Reactions Rate is NOT dependent on reactant concentration Graph of [A] vs. time gives STRAIGHT LINE If no straight line, reaction is NOT zero order Slope = -k Zero-Order Graph Integrated Rate Law Enables the determination a reactant’s concentration at any moment in time Enables the determination of the time it takes to reach a certain reactant concentration Enables the determination of the rate constant or reaction order st 1 Order Reactions Integrated Rate law ln[A]t – ln[A]0 = - kt ln[A] vs. time graph yields STRAIGHT LINE If no straight line, reaction is NOT 1st order Slope = -k st 1 Order Graph 1st Order Integrated Rate Law Only used with 1st order reactions Focus on initial concentration and ΔC for one reactant Initial concentration of reactant known---- can determine reactant concentration at any time Initial and final reactant concentrations known---can determine rate constant 1st Order Integrated Rate Law Rate = -Δ[A] = k [A] Δt -take equation and integrate with calculus to get…. ln[A]t – ln[A]0 = - kt [A]0 = initial concentration (t = 0) [A]t = concentration after a period of time Example 1: A B + 2D Using the data provided for a 1st order reaction, determine the rate constant and [A] at time = 5.0 x 102s. Time (s) [A] (M) 0 0.020 5.0 x 10 0.017 1.0 x 102 0.014 1.5 x 102 0.012 2.0 x 102 0.010 Example 1: continued Example 1: A B + 2D Using the data and graph provided, determine the rate constant and [A] at time = 5.0 x 102s. Time (s) [A] (M) 0 0.020 5.0 x 10 0.017 1.0 x 102 0.014 1.5 x 102 0.012 2.0 x 102 0.010 Half-life Radioactive decay is a 1st order process Half-life (t1/2)— Time it takes for half of a chemical compound to decay or turn into products Focus on reactant Constant, not dependent on [ ] Rate changes with temperature so half-life varies based on temperature Example 2: Find the half-life for the following reaction with a rate constant (k) of 1.70 x 10-3 s-1 nd 2 Order Reactions Integrated Rate Law 1___ - [A]t 1__ = kt [A]0 1/[A] vs. time graph yields STRAIGHT LINE If no straight line, reaction is NOT 2nd order Slope = k nd 2 Order Graph 2nd Order Integrated Rate Law Used only for second order reactions Focus on initial concentration and ΔC for one reactant with reaction 2nd order with respect to it. Initial concentration of reactant known---- can determine reactant concentration at any time Initial and final reactant concentrations known---can determine rate constant 2nd Order Integrated Rate Law Rate = -Δ[A] = k [A]2 Δt -take equation and integrate with calculus to get…. 1 __ [A]t 1__ = kt [A]0 [A]0 = initial concentration (t = 0) [A]t = concentration after a period of time Example 3: 2NO2(g) 2NO(g) + O2(g) Using the data provided, find the rate constant if the rate law = k[NO2]2. Time (s) [NO2] 0.0 0.070 1.0 x 102 0.0150 2.0 x 102 0.0082 3.0 x 102 0.0057 Example 3: 2NO2(g) 2NO(g) + O2(g) Using the data and graphs provided, find the rate law and rate constant. Time (s) [NO2] 0.0 0.070 1.0 x 102 0.0150 2.0 x 102 0.0082 3.0 x 102 0.0057 Example 3: continued Example 4: NO2 reacts to form NO and O2 by second-order kinetics with a rate constant = 32.6 L/molmin. What is the [NO2] after 1 minute if the initial [NO2] = 0.15M? Concentration and Time Data Use data to construct all graphs for zero, 1st, and 2nd reaction orders Determine which graph yields a straight line. [A] vs. Time Zero Order ln[A] vs. Time 1st Order 1/[A] vs. Time 2nd Order Homework Read over lab procedure








