final exam
advertisement

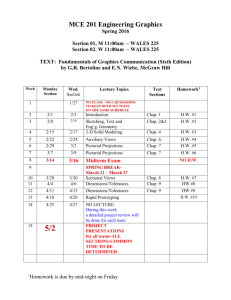
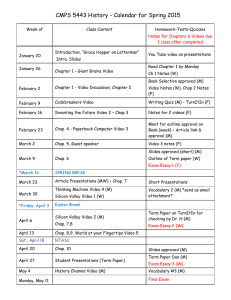
FINAL EXAM • Analogous to ACS Standardized Exam • 80 Multiple Choice • 120 minute time limit • No penalty for guessing Chap 9 Estimate ΔHrxn using bond energies (9.50) Chap 12 Calculate total heat for a series of phase transitions (12.72) Chap 13 Calculate molar mass from colligative property data (13.62) Chap 14 Derive a rate law from experimental data (14.17) • Chap 15 Calculate equilibrium concentrations (15.33) • Chap 17 Calculate a pH or pOH from concentration and Ka or Kb (17.10) • Chap 18 Calculate G for a process given the Ksp (18.25) • Chap 19 Determine the relative strengths of redox agents from E (Table 19.1) MECHANICS OF EXAM AND YOUR PREPARATION (1) The final is entirely multiple choice (80 questions) with approximately the partition of 90% qualitative and 10% quantitative. Although a choice of answers will be provided for the quantitative problems, you obviously must be able to perform the correct calculation in order to select the correct answer. (2) Do not memorize equations! I realized that we've seen a significant number. The intention of the exam is to determine how well you can manipulate data and reason logically. Data tables and any equations you may need will be provided. (3) Review of your old exams is the best method of preparation. Pay particular attention to those questions and problems that gave you difficulty the first time around and ensure that you can answer them now. (4) For qualitative prep, review your class notes and the Key Terms at the end of each chapter. Prep in this area lends itself very well to group study. (5) Quantitatively, choose several representative problems in each chapter and work them unassisted. If you are stumped, resort to your homework only to just get you past the obstacle. This method of "minimal assistance" is particularly effective! It’s analogous to having a “spotter” while you’re attempting to lift a heavy weight at the gym. Get the hint? See you 1200 Monday 26 Apr Here in the Sci Aud!! Several #2 pencils Calculator