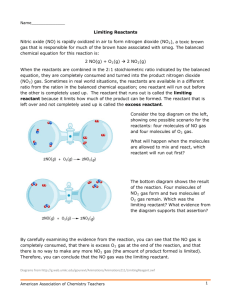
Limiting Reactants
advertisement

Limiting Reactants Chapter 13 Plan of the day • • • • Intro to Limiting Reactants S’mores Example Problems Work through homework problems – Ch 13 HW Assignment: #1-9, 12,13 • Chapter 13 Test: 5/4 Limiting Reactants—you will be able to…. (objectives) • Define a limiting reactant • Articulate the importance of a limiting reactant • Identify the limiting reactant in a chemical reaction • Calculate limiting reactant problems. Definitions • Reactant – Is a substance that takes part in a chemical reaction to make a product • Limiting Reactant – The reactant that is completely used up in the reaction and limits the amount of product • Excess Reactant – The reactant that is in excess, not all used up. Why do we care? (Don’t write… just listen) • Scientific Reasons – Determine how much reactant you need – Determine how much product will be produced • Economic (Show me the money!) – Keep production cost’s minimal – Maximize output in production S’mores Stoichiometry • Limiting Reactants • Complete S’mores wkst. #1-4 –We’ll get started together –When you are done, get a “S’mores Kit” S’mores Kit Stoichiometry • Record on back of your worksheet: 1. Obtain a bag with s’more ingredients 2. Write the balanced equation for S’mores! 3. Using Stoichiometry, determine how many S’mores you can make with each ingredient given. 4. What is your limiting reactant? 5. Using stoichiometry, determine the amount of your excess reactants. 6. Wash your hands, and make yourself a S’more as a reward for a job well done Steps in Solving LR problems! When two amounts of reactants are given in a problem, we need to identify the limiting reactant to solve for the amount of product possible! 1. 2. 3. 4. 5. Start with a Balanced Equation Change grams of reactants to moles – this is how much you have. Pick one of the reactants you just converted to mols (how much you have) & use Stoich from the balanced eqn to determine the number of mols of other reactant needed. Compare mols of other reactant needed to what you have of that reactant… do you have enough? Decide which is the limiting reactant Another Method 1. 2. 3. 4. Always begin with a BALANCED EQUATION!! Convert the amount (grams/volume) of each reactant to moles of reactants. Convert the number of moles of reactant to moles of product (doesn’t matter which one as long as you use the same product for both reactants) Which ever reactant produces the least product is the limiting reactant. Example 1 • The reaction begins with 2.51g of HF and 4.56g of SiO2. HF + SiO2 SiF4 + H2O • Balance Equation • Convert g mols of each (What you have) • Pick one & convert mols mols of other reactant. This is what you need…Do you have enough? • If “yes,” then the other is your LR… if no than that one is your LR • How much excess reactant is left over? – Determine the amount of the excess reactant used then subtract from the starting amount. How much product can be formed? • Start with the amount (g) of limiting reactant, use stoichiometry (balanced equation) to find out the amount (g) of product! Example 2 • Determine the limiting reactant, if an 80.0g solution of NaOH, which is 40.0% NaOH by mass, reacts with a 75.0g solution of H2SO4, which contains 45.0% water by mass. NaOH + H2SO4 Na2SO4 + H2O 1. Balance Equation 2. Calculate grams of NaOH and H2SO4 3. g mols of each (What you have) 4. Pick one & compare (What you need) 5. Do you have enough? • So… the reaction will go to completion until all the NaOH is used up. • Calculate the amounts of each product that will be produced. Reflection • Looking at the objectives, give me a finger vote. 1—still feel clueless 5— makes perfect sense! Example • If 40.0 g of H3PO4 react with 60.0 g of MgCO3 calculate: a. g of Mg3(PO4)2 produced b. g of CO2 produced c. Volume of CO2 at STP H3PO4 + MgCO3 Mg3(PO4)2 + CO2 + H2O IMPORTANT INFO… • Chapter 13 HW: – #1-9, 12,13 • Chapter 13 Test Dates –5/4/15 (A-day)