Differential Diagnosis Paper Group Paper for FNP Adult and
advertisement

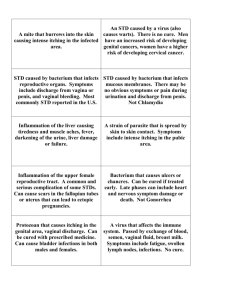
Running Head: DIFFERENTIAL DIAGNOSIS – VAGINAL DISCHARGE 1 Differential Diagnosis Associated with the Chief Complaint of Vaginal Discharge Presented to Dr. Cheryl Juneau ________________________________________ In Partial Fulfillment Of the Requirements for the Course GNRS 5669 FNP Adult/Women’s Health February 18, 2013 ________________________________________ By Kaci Burton and Penny Garner __________________________________________ THE UNIVERSITY OF TEXAS MEDICAL BRANCH AT GALVESTON SCHOOL OF NURSING 2 VAGINAL DISCHARGE Differential Diagnoses Associated with the Chief Complaint of Vaginal Discharge The purpose of this paper is to present a patient with the chief complaint of vaginal discharge. Based on the patient’s presentation, 5 differential diagnoses were developed: bacterial vaginosis, vaginal candidiasis, allergic vaginitis, gonorrhea, and chlamydia. These were narrowed down after reviewing the patient’s history and physical examination that are described in detail later. The 3 most likely diagnoses are bacterial vaginosis, vaginal candidiasis, and allergic vaginitis. Subjective L. B. is a 35 year-old white female that presents to the clinic with the complaint of vaginal discharge. She has been experiencing vaginal discharge for approximately 2-3 weeks. She explains that the vaginal discharge has been continuous throughout the day and night. She states that the discharge has remained consistent in quality and quantity throughout the 2-3 weeks and has a slight odor associated with it. She describes the discharge as a slight to moderate in quantity, thin, white, and mucous-like. The physical presentation regarding the vaginal discharge among the differentials are quite different. During the history taking a patient will describe the vaginal discharge as thin, white to gray in color, and malodorous with bacterial vaginosis, candidiasis is thick and curd-like, and allergic vaginitis is expected to be excessive and malodorous (Johnson, Thomas, & Porter, 2011). L.B. states that she first noticed the odor and discharge after having sex with her husband, and has not been sexually active since. She also admits to douching, as she always does, following her menstrual cycle 3 weeks ago. She denies using any new products for douching, such as perfumes or cleansers. An increase in odor following intercourse is indicative of bacterial vaginosis. VAGINAL DISCHARGE 3 Vaginal candidiasis and allergic vaginitis have no particular association with intercourse, except they may cause dyspareunia. Douching may lead to bacterial vaginosis, whereas douching with new fragrances or cleansers may cause allergic vaginitis (Hensley & Hollier, 2011). She has tried Monistat 1 day treatment 1 week ago without improvement or resolution. L.B. states that it is not affecting her work or normal daily activity, but it is affecting her sex life. She states that she is embarrassed and does not want to tell her husband what is going on until she finds out what the problem is. L.B. states that she is in generally good health and denies any significant medical history. She denies any surgeries, blood transfusions, serious accidents or injuries, or major illness. She reports that her only hospitalizations were for her 2 vaginal deliveries that were normal and without complications. She has no allergies to medications and currently takes a women’s multivitamin daily. She states that her vaccinations are up to date and has not received the influenza vaccine this season. She denies smoking or recreational drug use, but admits to drinking 2-3 glasses of wine per week and consuming 2 cups of coffee every morning before work. Her last complete physical exam was at her well-women 9 months ago, but her primary care physician saw her 4-5 months ago for a “cold”. Her last PAP was 9 months ago and has no history of any abnormal results. She has not yet started getting mammograms, but she does do selfbreast exams when she thinks about it. L.B. states that she does not use any type of birth control method since her husband had a vasectomy 4 years ago. The introduction of condoms, spermicide, or lubricants leads to allergic vaginitis resulting in inflammation and edema (Dains, Baumann, & Sheibel, 2012). She is the primary cook in the home and tries to prepare well-balanced meals, but states she eats worse at work. She walks VAGINAL DISCHARGE 4 in her neighborhood approximately 3-4 days per week for 30-45 minutes with her husband and children. L.B. has been married for 10 years and lives in a single-story home with her husband and 2 children. She has a son that is 9 years old and a daughter that is 5 years old. She works in the human resource department at Sam’s Club. She does not express any excess stress, economic concerns or religious considerations that may affect her care. L.B. describes her family as being in generally good health. On her maternal side she has a grandmother with hypertension (HTN) and depression, an aunt with infertility issues (unknown specifics), and her mother suffers with depression and restless leg syndrome. On her paternal side she only knows that her father has HTN and chronic back pain. The patient’s view and impression on her condition was reviewed by utilizing the patient explanatory model. L.B. expresses general concern and embarrassment about her current condition. She explains, “I thought it was a yeast infection because I get those every now and then. I went to Walgreens and bought some Monistat and when it did not help my problem I became worried. I don’t think it’s an STD, because my husband and I are faithful to each other, but I have no idea what it could be. I just want the discharge to be cleared up because it is embarrassing”. L.B. describes herself as being in generally good health with no recent major illnesses or need for antibacterial therapy. She has not had any weight loss or gain in over a year. She reports her diet as regular with no specific restrictions. L.B denies any skin lesions, masses, lumps, or nodules. She also denies any swelling or tenderness of 5 VAGINAL DISCHARGE the lymph nodes. She denies any head or neck pain as well as denies oral lesions or pain in the mouth. L.B. denies ever having any chest pain, palpitations, or swelling in the extremities. She states she never experiences shortness of breath or has any unusual cough. L.B. reports no abdominal pain. She describes her gynecological history as having two normal, uncomplicated vaginal deliveries, she douches after every menstrual cycle, and her last menstrual cycle was three weeks ago. L.B. confirms a malodorous vaginal discharge for approximately 2-3 weeks of thin, white, mucous-like consistency in minimal to moderate amounts. She denies any burning, painful, frequent, or malodorous urination. She also denies and pruritus in the vaginal area. She describes her mental state as “somewhat depressed about this discharge.” Objective L.B. can be described as an alert, oriented, well-dressed, well-nourished middleaged white female in no acute distress. Her expression is that of nervousness and seems genuinely concerned about the reason for her office visit. Her left arm blood pressure is 118/62 with a regular radial heart rate of 72. Her respirations are 18 and is afebrile with an oral temperature of 97.2 degrees F. She is 5’ 8” tall and weighs 130 pounds which is equivalent to a body mass index of 19.8 kg/m2. L.B.’s skin is warm and dry with no rashes, lesions, or skin breakdown present. Her head is normocephalic with no lesions present. Her neck is supple with trachea midline. No lymph node swelling or tenderness noted on palpation. The thyroid gland is supple without enlargement, nodules or bruit. L.B.’s heart has normal S1, S2 with no murmurs, rubs, or gallops. The capillary refill on L.B. is <3 seconds. The patient’s respirations are even and unlabored with symmetrical chest expansion. Her lungs are clear to auscultation in VAGINAL DISCHARGE 6 all lobes anteriorly and posteriorly. L.B has a symmetrical abdomen that is soft, round, and nontender on palpation without masses or organomegaly. Her bowel sounds are active over all quadrants. Upon inspection, the patient’s external genitalia is normal in appearance. There is mild irritation and redness noted around the vulva without lesions. An inspection of the folds of the labia majora, labia minora, and urethra reveal no redness or lesions. The Bartholin and Skene glands are not palpable. Upon speculum exam of the vagina there is noted a moderate amount of thin, grey-white discharge that is mildly adherent to the vaginal walls with a fishy odor. No mucopurulent or curd-like discharge noted. The cervix is pink and closed with no lesions visible and no cervical motion tenderness. The perianal area is normal with no lesions noted. Her uterus is palpable on bimanual exam and is small and soft with no adnexal or cervical motion tenderness (CMT) noted. Ovaries are not palpable. No symptoms alone or findings on physical examination are very conclusive for vaginal infections, therefore microscopy is most beneficial in definitively diagnosing these infections when classic findings are present (Domino, 2012). In suspicion of bacterial vaginosis vs. vaginal candidiasis vs. allergic vaginitis it is decided during the speculum exam of the vagina to take a swab sample of the discharge from the lateral fornices in order to prepare several wet mounts for microscopic exam. Two glass slides are prepared with a smear of the vaginal discharge applied, one using 10% potassium hydroxide (KOH) and the other using normal saline (NaCl or NS). The whiff test is positive to the KOH slide and the microscope exam reveals no budding hyphae. It is only in BV that the whiff test will be positive and only in vaginal candidiasis will there be budding hyphae present (Dains et al, 2012). This KOH slide does not rule out allergic 7 VAGINAL DISCHARGE vaginitis. The NS wet mount is viewed under the microscope next and is positive for clue cells, and few lactobacilli. No WBC’s are seen on this slide. This finding on the NS wet mount is consistent in BV. This slide rules out allergic vaginitis as you would see the presence of WBC’s on a NS wet mount (Dains et al, 2012). The vaginal discharge is then applied to pH paper with a reading of 5.2. This test rules out both vaginal candidiasis and allergic vaginitis because only in BV is the pH > 4.5 (Dains et al, 2012). Assessment 1. Bacterial Vaginosis The medical diagnosis for L.B. is bacterial vaginosis. Bacterial vaginosis (BV) is the most common cause of vaginal discharge and most commonly found in reproductive age women (Dains et al, 2012). BV is a disturbance of the normal flora in the vagina caused when hydrogen peroxide producing lactobacilli are replaced by other bacteria, usually anaerobes. BV is characterized by a thin white, grey, green, or brownish discharge that is malodorous and moderately adherent to the vaginal wall. Pelvic tenderness may be present, but no CMT. Fifty percent of women are asymptomatic, but most exhibit a musty or fishy vaginal odor, commonly immediately after intercourse. The most clinically significant diagnostic test for BV is the presence of clue cells on a NS wet mount. The Amsel criteria for the diagnosis of BV requires that 3 out of 4 indicators be present: presence of clue cells on a NS wet mount, presence of characteristic vaginal discharge, pH > 4.5, and a positive whiff test. (Domino, 2012). L.B.’s presentation confirms the medical diagnosis of BV because of her characteristic vaginal discharge as that of thin, white and having an odor noticed after having intercourse with her husband. Upon speculum exam of the vagina it is noted the VAGINAL DISCHARGE 8 discharge is moderately adherent to the vaginal walls. Upon bimanual exam there is no CMT noted. Diagnostic tests confirm BV as the medical diagnosis due to the presence of clue cells and WBC’s on microscopic exam of the vaginal discharge. L.B. has 4 of 4 indicators required in the Amsel clinical criteria to confirm the diagnosis of BV. 2. Vaginal candidiasis Vaginal candidiasis occurs when an alteration in the balance of microorganisms in the vagina allow for growth of yeast. The most common organism is candida albicans. This condition accounts for 33% of all vaginal infections and occurs most commonly in women from menarche to menopause. Risk factors for this infection include recent antibiotic use, an immunocompromised state, hypothyroidism, diabetes, anemia, use of oral contraceptives, wearing tight fitting clothing, previous candidal infection, and obesity. The patient presentation will include a vaginal discharge that is thick, white, curd-like and may have white patches on the vaginal mucosa. The discharge does not have an odor. She will likely be experiencing vulvar itching, erythema, dyspareunia, and dysuria. The presence of budding yeast and pseudohyphae on a KOH wet mount and a vaginal pH of < 4.5 would also confirm the diagnosis of vaginal candidiasis. (Hensley & Hollier, 2011). The diagnosis of vaginal candidiasis is ruled out because L.B.’s discharge is not characteristic of thick and curd-like and it has an odor. She denies complaints of vaginal itching, dyspareunia and dysuria. The KOH wet mount prepared with L.B.’s vaginal discharge did not have any budding yeast or pseudohyphae and the pH of her vaginal secretions was > 4.5. 3. Allergic Vaginitis 9 VAGINAL DISCHARGE A substance that comes into direct contact with the vulvovaginal area is generally the cause of allergic vaginitis. The causative allergen is usually something that has been introduced recently such as vaginal lubricants, douches, spermicides, and or condoms. These particular substances may cause irritation, inflammation, and edema in some cases, which leads to the allergic vaginitis (Dains et al, 2012). The patient may present with a malodorous excessive vaginal discharge, pruritus, and dysuria (Johnson, Thomas, & Porter, 2011). This diagnosis was considered a possibility based on the patient’s presentation and feminine hygiene practices. L.B. complained of an odorous vaginal discharge that she subsequently noticed after intercourse with her husband. Around the same time she had just finished her menstrual cycle and douches afterwards. The diagnosis of allergic vaginitis was ultimately ruled out because L.B. did not have dysuria and denied douching with a new cleaning solution. In addition to the lack of new allergen exposure, the patient also had no erythema or edema noted on external inspection or speculum exam. Lastly, the bedside pH strip testing revealed a vaginal pH greater than 4.5, unlike allergic vaginitis, which causes a more acidic environment with a pH less than 4.5 (Dains et al, 2012). Plan The diagnostic labs indicated for the chief complaint and presentation of vaginal discharge by L.B. are the wet mount and pH strip. Both of these tests were performed in the office during L.B.’s visit. Vaginal cultures were considered, but decided not necessary because both the wet mounts and pH strip were conclusive for the diagnosis of BV. See Appendix A for further information on the diagnostic lab results. No further VAGINAL DISCHARGE 10 diagnostics are indicated at this time. First line treatment for BV is Metronidazole vaginal gel: 0.75% 5 grams intravaginally daily x 7 days (Domino, 2012). The prescription for Metronidazole was written for L.B. for 7 doses with no refills. Education was provided to L.B. which included all of the following: Metronidazole produces a disulfiramlike effect when alcohol is ingested. A disulfiramlike effect is characterized by severe flushing and may include tachycardia and hypotension. Avoid any alcoholcontaining product while taking metronidazole. Avoid use of panty liners, pantyhose, and occlusive pants and undergarments. Avoid douching to prevent recurrence. Delay sexual relations until symptoms clear, discomfort resolves, and treatment is complete (Domino, 2012). Informed patient of the websites ACOG.org and CDC.gov and that she may find additional helpful information here to educate herself. There is no indication for a referral based on L.B.’s presentation and diagnosis. L.B. was informed that no specific follow-up is needed, but if symptoms persist or recur within 2 months, to make another appointment for a repeat pelvic exam and possible vaginal cultures. L.B. verbalized complete understanding of all instructions given. 11 VAGINAL DISCHARGE References Dains, J., Baumann, L., & Scheibel, P. (2012). Advanced health assessment and clinical diagnosis in primary care. St. Louis: Elsevier Mosby. Domino, F.J. (2012). The 5-minute clinical consultant (20th ed.). Philadelphia, PA: Lippincott Williams & Wilkins. Hensley, R. & Hollier, A. (2011). Clinical guidelines in primary care: A reference and review book. Lafayette: Advanced Practice Education Associates, Inc. Johnson, J., Thomas, D., & Oscar, B. (2011). Women’s health problems. In L.M. Dunphy, J.E. Winland-Brown, B.O. Porter, & D.J. Thomas (Eds.), Primary care: The art and science of advanced practice nursing (pp. 661-734). Philadelphia, PA: F.A. Davis Company. 12 VAGINAL DISCHARGE Appendix A Vaginal Discharge Differential Diagnosis Table Subjective Data Bacterial Vaginosis Vaginal Candidiasis Allergic Vaginitis +Vaginal discharge, +Vaginal discharge, +Vaginal discharge, increased odor dysuria, pruritic Introduction of after intercourse, discharge, new allergens: no dyspareunia, dyspareunia, recent bubble bath, recent change in use of antibiotics lubricants, or sexual partner Objective Data douche Thin white to gray Copious, thick, Mild to moderate malodorous vaginal white, curd-like malodorous vaginal discharge, vaginal discharge, discharge, Edema and Labia are inflammation and erythema erythematous and edema edematous, reddened vaginal wall Lab/Diagnostic Data Vaginal pH >4.5, Vaginal pH 4-5 Vaginal pH <4.5 Wet mount: +clue Wet mount: Wet mount: +WBC cells, +KOH amine +pseudophyphae test “whiff test” & branching yeast (Dains et al, 2012).