chem 102 - Louisiana Tech University
advertisement

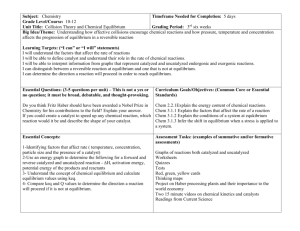
Chemistry 102 Fall 2015 Instructor: Dr. Upali Siriwardane e-mail: upali@latech.edu Office: CTH 311 Phone 257-4941 Office Hours: M.W &F, 8:00-9:00 & 11:00-12:00 and Tu ,Th 8:00 - 10:00 am. am. or by appointment Test Dates Sept. 29, 2015 (Test 1): Chapter 13 Oct. 22, 2015 (Test 2): Chapter 14 &15 Nove. 17, 2015 (Test 3): Chapter 16 &17 Nvember 19, 2015 (Make-up test) comprehensive: Chapters 13-17 CHEM 102 Fall 15, LA TECH 14-2-1 Chapter 14. Chemical Equilibrium 14.1 14.2 14.3 14.4 Fetal Hemoglobin and Equilibrium 61 3 The Concept of Dynamic Equilibrium 61 5 The Equilibrium Constant (K) 61 8 Expressing the Equilibrium Constant in Terms of Pressure 622 14.5 Heterogeneous Equilibria: Reactions Involving Solids and Liquids 625 14.6 Calculating the Equilibrium Constant from Measured Equilibrium Concentrations 626 14.7 The Reaction Quotient: Predicting the Direction of Change 629 14.8 Finding Equilibrium Concentrations 631 14.9 Le Châtelier’s Principle: How a System at Equilibrium Responds to Disturbances 641 CHEM 102 Fall 15, LA TECH 14-2-2 Law of mass Action Defines an equilibrium constant (K) for the process jA+kB lC+mD [C]l[D]m K = ----------------- ; [A], [B] etc are [A]j[B]k Equilibrium concentrations Pure liquid or solid concentrations are not written in the expression. CHEM 102 Fall 15, LA TECH 14-2-3 7) What is the difference between initial [A]i and equilibrium [A]eq concentrations? N2O4(g) colorless Initial N2O4 NO2 0.00 0.02 0.00 0.03 0.00 0.04 0.02 0.00 CHEM 102 Fall 15, LA TECH [NO2]2 Keq [N2O4 ] @ Equilibrium N2O4 NO2 0.0014 0.017 0.0028 0.024 0.0045 0.031 0.0045 0.031 2NO2(g) Dark brown Keq 0.21 0.21 0.21 0.21 14-2-4 Equilibrium calculations We can predict the direction of a reaction by calculating the reaction quotient. Reaction quotient, Q For the reaction: aA + bB eE + fF [E]e [F]f Q = [A]a [B]b Q has the same form as Kc with one important difference. Q can be for any set of concentrations, not just at equilibrium. CHEM 102 Fall 15, LA TECH 14-2-5 Equilibrium constant calculations 1) Consider the reaction: COCl2(g) CO(g) + Cl2(g) At equilibrium, [CO] = 4.14 × 10-6 M; [Cl2] = 4.14 × 10-6 M; and [COCl2] = 0.0627 M. Calculate the value of the equilibrium constant. CHEM 102 Fall 15, LA TECH 14-2-6 Terminology Initial concentration: concentration (M) of reactants and products before the equilibrium is reached. Equilibrium Concentration Concentration (M) of reactants and products After the equilibrium is reached. CHEM 102 Fall 15, LA TECH 14-2-7 Reaction quotient Any set of concentrations can be given and a Q calculated. By comparing Q to the Kc value, we can predict the direction for the reaction. Q < Kc Q = Kc Q > Kc Net forward reaction will occur. No change, at equilibrium. Net reverse reaction will occur. CHEM 102 Fall 15, LA TECH 14-2-8 Reaction quotient (Q) calculations 2) Consider the reaction system: C2H5OH (aq) + CH3COOH(aq) CH3COOC2H5 +H2O (l) [ethyl acetate]eq 𝐊= = 𝟎. 𝟗𝟓 [ethanol] eq[acetic acid] eq 𝐐= [ethyl acetate]in =? [ethanol] in[acetic acid] in The concentrations of both ethanol and acetic acid are 0.45 M and the concentration of ethyl acetate is 1.1 M. Use the reaction quotient to determine whether the system is at equilibrium. CHEM 102 Fall 15, LA TECH 14-2-9 Reaction quotient (Q) calculations Consider the reaction system: C2H5OH (aq) + CH3COOH(aq) CH3COOC2H5 +H2O (l) The concentrations of both ethanol and acetic acid are 0.45 M and the concentration of ethyl acetate is 1.1 M. Use the reaction quotient to determine whether the system is at equilibrium. [ethyl acetate]in 𝐐= =? [ethanol] in[acetic acid] in CHEM 102 Fall 15, LA TECH and Keq=0.95 14-2-10 Determining Equilibrium Constants ICE Method 1. Derive the equilibrium constant expression for the balanced chemical equation 2. Construct a Reaction Table with information (ICE) about reactants and products 3. Include the amounts reacted, x, in the Reaction Table 4. Calculate the equilibrium constant in terms of x CHEM 102 Fall 15, LA TECH 14-2-11 Example: An equilibrium is established by placing 2.00 moles of N2O4(g) in a 5.00 L and heating the flask to 407 K. It was determined that at equilibrium the concentration of the NO2(g) is 0.525 mol/L. What is the value of the 2 [NO ] 2 equilibrium constant? Kc = [N2O4] N2O4(g) 2 NO2(g) N2O4(g) [Initial] (mol/L) [Change] 0.40 -x 0 2x -1/2 x x [Equilibrium] 0.40 - 1/2x 0.40- 0.263= 0.138 CHEM 102 Fall 15, LA TECH 2 NO2(g) =0+x 0.525 14-2-12 What is the value of the equilibrium constant? N2O4(g) [NO2]2 Kc = [N2O4] 2 NO2(g ) 0.525 = 0 + x x = 0.525 0.40 - 1/2x [NO2] = 0.40 - 1/2x = 0.40 - 1/2(0525) Kc = [NO2]2 [N2O4] CHEM 102 Fall 15, LA TECH = (0.525)2 = 2.00 0.138 14-2-13 Equilibrium Calculations Hydrogen iodide, HI, decomposes according to the equation 2 HI(g) H2(g) + I2(g) When 4.00 mol of HI placed in a 5.00-L vessel at 458ºC, the equilibrium mixture was found to contain 0.442 mol I2. What is the value of Kc for the reaction? CHEM 102 Fall 15, LA TECH 14-2-14 2 HI(g) Initial Change H2(g) + I2(g) 4.00/5=.80 0 0 -2x x x Equilibrium 0.80-2x x x=0.442/5 x = 0.0884 Equilibrium concentrations [HI] = 0.80 - 2x = 0.8 - 2 x 0.0884 = 0.62 [H2] = x = 0.0884 [I2] = x = 0.0884 [H2] [I2] 0.0884 x 0.0884 Kc = ---------------- = ------------------------- = 0.0201 [HI]2 (0.62) 2 CHEM 102 Fall 15, LA TECH 14-2-15 Equilibrium Calculation Example A sample of COCl2 is allowed to decompose. The value of Kc for the equilibrium COCl2 (g) CO (g) + Cl2 (g) is 2.2 x 10-10 at 100 oC. If the initial concentration of COCl2 is 0.095M, what will be the equilibrium concentrations for each of the species involved? CHEM 102 Fall 15, LA TECH 14-2-16 Equilibrium Calculation Example COCl2 (g) Initial conc., M 0.095 Change -X in conc. due to reaction CO (g) Cl2 (g) 0.000 0.000 +X +X Equilibrium M (0.095 -X) X Concentration, [ CO ] [ Cl2 ] Kc = = [ COCl2 ] CHEM 102 Fall 15, LA TECH X X2 (0.095 - X) 14-2-17 Equilibrium calculation example Keq = 2.2 x 10-10 = X2 (0.095 - X) Rearrangement gives X2 + 2.2 x 10-10 X - 2.09 x 10-11 = 0 a X2 + b X - c = 0 This is a quadratic equation. Fortunately, there is a straightforward equation for their solution CHEM 102 Fall 15, LA TECH 14-2-18 Quadratic Equations An equation of the form a X2 + b X + c = 0 Can be solved by using the following x= -b + 2 b - 4ac 2a Only the positive root is meaningful in equilibrium problems. CHEM 102 Fall 15, LA TECH 14-2-19 Equilibrium Calculation Example 2 X + 2.2 x 10 a b 2 X = X= -b + b 2a - 2.2 x 10 X = 4.6 x 10 -6 X = -4.6 x 10 -10 -10 X - 2.09 x 10 -11 =0 c - 4ac -10 2 -11 1/2 + [(2.2 x 10 ) - (4)(1)(- 2.09 x 10 )] 2 M -6 M CHEM 102 Fall 15, LA TECH 14-2-20 Equilibrium Calculation Example Now that we know X, we can solve for the concentration of all of the species. COCl2 CO Cl2 = 0.095 - X = X = X = 0.095 M = 4.6 x 10-6 M = 4.6 x 10-6 M In this case, the change in the concentration of is COCl2 negligible. CHEM 102 Fall 15, LA TECH 14-2-21 Equilibrium calculations using ICE 3) Consider the reaction A 2B, where the value of Keq is 1.4 × 10-12. At equilibrium, the concentration of B is 0.45 M. What is the concentration of A? ICE Calculation [A] [B] Initial concentration: Change Equilibrium concentration: K = 1.4 x 10-12 CHEM 102 Fall 15, LA TECH 14-2-22 Equilibrium calculations using ICE 4) Calculation of unknown concentration of reactants or products in an equilibrium mixture At 100o C the equilibrium constant (K) for the reaction: H2(g) + I2(g) 2 HI(g) ; K = 1.15 x 102. If 0.400 moles of H2 and 0.400 moles. If I2 are placed into a 12.0-liter container and allowed to react at this temperature, what is the HI concentration (moles/liter) at equilibrium? ICE Calculation [H2] [I2] [HI] Initial concentration: Change CHEMEquilibrium 102 Fall 15, LA TECH concentration: 14-2-23 Equilibrium calculations using ICE 4) H2(g) + I2(g) 2 HI(g) ; K = 1.15 x 102. If 0.400 moles of H2 and and I2 0.400 moles each are placed into a 12.0-liter container and allowed to react at this temperature, what is the HI concentration (moles/liter) at equilibrium? ICE Calculation Initial concentration: Change Equilibrium concentration: K = 1.15 x 102= [H2] 0.033 -x 0.033-x [I2] 0.033 [HI] 0 -x 2x 0.033-x 2x= 0.0281 x 2 =0.056 [HI] 2 𝟐𝒙 𝟐𝒙 =( )2;𝐊 = ; 10.72=2x/(0.033-x) 𝟎.𝟎𝟑𝟑−𝒙 𝟎.𝟎𝟑𝟑−𝒙 [I2] [H2] (10.72 x 0.033)-10.72x =2x; (0.357)-10.72x =2x; -12.72x =-.357; x = 0.0281 CHEM 102 Fall 15, LA TECH 14-2-24 Equilibrium calculations using ICE 4) H2(g) + I2(g) 2 HI(g) ; K = 1.15 x 102. If 0.400 moles of H2 and 0.400 moles. If I2 are placed into a 12.0-liter container and allowed to react at this temperature, what is the HI concentration (moles/liter) at equilibrium? ICE Calculation [H2] [I2] [HI] Initial concentration: Change Equilibrium concentration: K = 1.15 x 102 CHEM 102 Fall 15, LA TECH 14-2-25 What is K (Kc) and Kp Kc (K) - equilibrium constant calculated based on [A]-Concentrations. Kp- equilibrium constant calculated based on partial pressure Kp = CHEM 102 Fall 15, LA TECH 14-2-26 Pressure Equilibrium Constants Kc & Kp N2 + 3H2 Kc = Kc = = [NH3]2 [N2][H2]3 2NH3 (PNH3/RT)2 = (PN2/RT) (PH2/RT)3 (PNH3)2 (1/RT)2 (PN2) (1/RT))(PH2)3(1/RT)3) PNH32 PN2 PH23 CHEM 102 Fall 15, LA TECH (1/RT)2 (1/RT)(1/RT)3 = Kp (1/RT) 2 (1/RT)(1/RT)3 14-2-27 Kc vs. Kp N2 (g) + 3H2 (g) K c = Kp 2NH3 (g) (1/RT)2 )-2 = K (1/RT p 3 (1/RT)(1/RT) In General Kc = Kp (1/RT)Dn Kc = Kp (RT)-Dn where Dn = #moles gaseous products - # moles gaseous reactants CHEM 102 Fall 15, LA TECH 14-2-28 What is K (Kc) and Kp Kc (K) - equilibrium constant calculated based on [A]-Concentrations. Kp- equilibrium constant calculated based on partial pressure (p) Kc = Kp(RT)-Dn Kp = Kc(RT) Dn atm L R = universal gas constant (0.08206 mol K ) T = Kelvin Temperature, Dn = (sum of stoichiometric coefficients of gaseous products) - (sum of the stoichiometric coefficients of gaseous reactants) CHEM 102 Fall 15, LA TECH 14-2-29 Partial pressure & Equilibrium Constants For the following equilibrium, Kc = 1.10 x 107 at 700. oC. What is the Kp? 2H2 (g) + S2 (g) Kp = Kc (RT)Dng T = 700 + 273 = 973 K 2H2S (g) L R = 0.08206 atm mol K Dng = ( 2 ) - ( 2 + 1) = -1 CHEM 102 Fall 15, LA TECH 14-2-30 Partial pressure & Equilibrium Constants Kp = Kc Dn (RT) g [ = 1.10 x 107 (0.08206 = 1.378 x105 CHEM 102 Fall 15, LA TECH atm L mol K ) (973 K) ] -1 14-2-31 Types of Equilibrium 1) Heterogeneous Equilibrium 2) Heterogeneous Equilibrium 3) Acid Dissociation Constant- Ka 4) Base Dissociation Constant- Kb 5) Autoionization Constant- Kw 6) Solubility Product Constant-Ksp CHEM 102 Fall 15, LA TECH 14-2-32 Heterogeneous Equilibrium CaCO3(s) CaO(s) + CO2(g) [CaO(s)][CO2(g)] Kc = [CaCO3(s)] concentrations of pure solids and liquids are constant are dropped from expression Kc = [CO2(g)] CHEM 102 Fall 15, LA TECH 14-2-33 Solubility Product of Salts in Water AgCl(s) + H2O (l) Ag+(aq) + Cl- Ksp = [Ag+] [Cl-] Ksp (AgCl) = 1.77 × 10-10 Ksp (BaSO4) = 1.1 x 10-10 CHEM 102 Fall 15, LA TECH 14-2-34 Acid Dissociation Constant HC2H3O2 (aq) + H2O(l) K = H3O+ (aq) + C2H3O2- (aq) [H3O+][C2H3O2-] [H2O][HC2H3O2] Ka = K [H2O] = CHEM 102 Fall 15, LA TECH [H3O+][C2H3O2-] [HC2H3O2] 14-2-35 Base Dissociation Constant NH4+ + OH- NH3 + H2O(l) K = [NH4+][OH-] [H2O][NH3] Kb = K [H2O] = CHEM 102 Fall 15, LA TECH [NH4+][OH-] [NH3] 14-2-36 Autoionization of Water H2O (l) + H2O (l) K = H3O+ + OH- [H3O+][OH-] [H2O]2 Kw = K [H2O]2 = [H3O+][OH-] = 1.0 10-14 CHEM 102 Fall 15, LA TECH 14-2-37 What is the reaction quotient, Q (Q) is constant in the equilibrium expression when initial concentration of reactants and products are used. SO2(g)+ NO2(g) NO(g) +SO3(g) [NO][SO3] Q = ---------------[SO2][NO2] comparing to K and Q provide the net direction to achieve equilibrium. CHEM 102 Fall 15, LA TECH 14-2-38 Predicting the Direction of a Reaction CHEM 102 Fall 15, LA TECH 14-2-39 Q Calculation Consider the following reaction: SO2(g) + NO2(g) NO(g) + SO3(g) (Kc = 85.0 at 460oC) Given: 0.040 mole of SO2(g), 0.500 mole of NO2(g), 0.30 mole of NO(g),and 0.020 mole of SO3(g) are mixed in a 5.00 L flask, Determine: a) The net the reaction quotient, Q. b) Direction to achieve equilibrium at 460oC. CHEM 102 Fall 15, LA TECH 14-2-40 Q Calculation SO2(g) + NO2(g) NO(g) + SO3(g) (Kc = 85.0 at 460oC) [NO][SO3] Q = ------------[SO2][NO2] 0.040 mole 0.500 mole 0.30 mole 0.020 mole [SO2] = -------------; [NO2] = ----------- ; [NO] = ------------; [SO3] = ----------5.00 L 5.00L 5.00L 5.00 L [SO2] = 8 x 10-3mole/L ; [NO2] =0.1mole/L; [NO] = 0.06 mole/L; [SO3] = 4 x 103mole/L Q= 0.06 (4 x 10-3 ) ---------------------8.0 x 10-3 x 0.1 = 0.3 Therefore the equilibrium shift to right CHEM 102 Fall 15, LA TECH 14-2-41 Le Chatelier’s principle Any stress placed on an equilibrium system will cause the system to shift to minimize the effect of the stress. You can put stress on a system by adding or removing something from one side of a reaction. N2(g) + 3H2 (g) 2NH3 (g) What effect will there be if you added more ammonia? How about more nitrogen? CHEM 102 Fall 15, LA TECH 14-2-42 Predicting Shifts in Equilibria Equilibrium concentrations are based on: • The specific equilibrium • The starting concentrations • Other factors such as: • Temperature • Pressure • Reaction specific conditions Altering conditions will stress a system, resulting in an equilibrium shift. CHEM 102 Fall 15, LA TECH 14-2-43 Increase in Concentration or Partial Pressure for N2(g) + 3 H2(g) 2 NH3(g) an increase in N2 and/or H2 concentration or pressure, will cause the equilibrium to shift towards the production of NH3 CHEM 102 Fall 15, LA TECH 14-2-44 Shifts with Temperature N2O4(g) colorless 2NO2(g) Dark brown N2O4(g) 2 NO2(g) ; D H=? (+or -) CHEM 102 Fall 15, LA TECH 14-2-45 Predicting Equilibrium Shifts For the following equilibrium reactions: H2(g) + CO2(g) H2O(g) + CO(g) DH = 40 kJ Predict the equilibrium shift if: a) The temperature is increased b) The pressure is decreased CHEM 102 Fall 15, LA TECH 14-2-46 Shifting of Equilibrium N2O4(g) 2 NO2(g) CHEM 102 Fall 15, LA TECH 14-2-47 Changes in pressure In general, increasing the pressure by decreasing volume shifts equilibrium towards the side that has the smaller number of moles of gas. Unaffected by pressure H2 (g) + I2 (g) 2HI (g) Increased pressure, shift to left N2O4 (g) CHEM 102 Fall 15, LA TECH 2NO2 (g) 14-2-48 5) How you would increase the products of following industrially important reactions: a) CO(g) + H2O (g) H2 (g) + CO2 (g);DH= −41.2 kJ/mol b) N2(g) + 3H2(g) nitrogen hydrogen CHEM 102 Fall 15, LA TECH 2NH3(g) H = -92.4 kJ mol-1 ammonia 14-2-49 5) How you would increase the products of following industrially important reactions: a) CO(g) + H2O (g) H2 (g) + CO2 (g);DH= −41.2 kJ/mol Pressure: no change in Dn, pressure have no effect: moderate pressure Temperature: DH is – (exothermic) heats up: moderate temperature Catalysts: Increase the rate at which equilibrium is reached b) N2(g) + 3H2(g) 2NH3(g) H = -92.4 kJ mol-1 nitrogen hydrogen ammonia Pressure: change in Dn= -2, pressure shift to more NH3: higher pressure. Temperature: DH is – (exothermic) heats up: moderate pressure Catalysts: Increase the rate at which equilibrium is reached CHEM 102 Fall 15, LA TECH 14-2-50 Equilibrium Systems product-favored if K > 1 exothermic reactions favor products increasing entropy in system favors products at low temperature, product-favored reactions are usually exothermic at high temperatures, product-favored reactions usually have increase in entropy CHEM 102 Fall 15, LA TECH 14-2-51 Thermodynamics of Equilibrium a) Enthalpy (DH) b) Entropy (DS) c) Free Energy (DG) (D G is a combined term involving DH, DS and T) CHEM 102 Fall 15, LA TECH 14-2-52 Probability, Entropy and Chemical Equilibrium CHEM 102 Fall 15, LA TECH 14-2-53 Entropy measure of the disorder in the system more disorder for gaseous systems than liquid systems, more than solid systems Chapter 18. Thermodynamics DG = DH -TDS DG = Gibbs Free Energy (- for spontaneous) DH = Enthalpy DS = Entropy T = Kelvin Temperature CHEM 102 Fall 15, LA TECH 14-2-54 Equilibrium Reaction Rates reactions occur faster in gaseous phase than solids and liquids reactions rates increase as temperature increases reactions rates increase as concentration increases rates increase as particle size decreases rates increase with a catalyst CHEM 102 Fall 15, LA TECH 14-2-55 Industrial Production of Ammonia catalysis N2(g) + 3 H2(g) 2 NH3(g) ; DH = - high pressure and temperature CHEM 102 Fall 15, LA TECH 14-2-56 Ammonia Synthesis reaction is slow at room temperature, raising temperature, increases rate but lowers yield increasing pressure shifts equilibrium to products liquefying ammonia shifts equilibrium to products use of catalyst increases rate CHEM 102 Fall 15, LA TECH 14-2-57 Haber-Bosch Process CHEM 102 Fall 15, LA TECH 14-2-58 Decrease in Concentration or Partial Pressure for N2(g) + 3 H2(g) 2 NH3(g) ; DH = - likewise, a decrease in NH3 concentration or pressure will cause more NH3 to be produced CHEM 102 Fall 15, LA TECH 14-2-59 Changes in Temperature for N2(g) + 3 H2(g) 2 NH3(g) ; DH = - for an exothermic reaction, an increase in temperature will cause the reaction to shift back towards reactants and vice versa. CHEM 102 Fall 15, LA TECH 14-2-60 Volume Change for N2(g) + 3 H2(g) 2 NH3(g) ; DH = - an increase in volume, causes the equilibrium to shift to the left where there are more gaseous molecules a decrease in volume, causes the equilibrium to shift to the right where there are fewer gaseous molecules CHEM 102 Fall 15, LA TECH 14-2-61 Shifts with Temperature N2O4(g) colorless 2NO2(g) Dark brown N2O4(g) 2 NO2(g) ; D H=? (+or -) CHEM 102 Fall 15, LA TECH 14-2-62 Probability, Entropy and Chemical Equilibrium CHEM 102 Fall 15, LA TECH 14-2-63 Entropy measure of the disorder in the system more disorder for gaseous systems than liquid systems, more than solid systems Chapter 17. Thermodynamics DG = DH -TDS DG = Gibbs Free Energy (- for spontaneous) DH = Enthalpy DS = Entropy T = Kelvin Temperature CHEM 102 Fall 15, LA TECH 14-2-64 Predicting Equilibrium Shifts For the following equilibrium reactions: H2(g) + CO2(g) H2O(g) + CO(g); DH = 40 kJ Predict the equilibrium shift if: a) The temperature is increased b) The pressure is decreased CHEM 102 Fall 15, LA TECH 14-2-65 Equilibrium Systems product-favored if K > 1 exothermic reactions favor products increasing entropy in system favors products at low temperature, product-favored reactions are usually exothermic at high temperatures, product-favored reactions usually have increase in entropy CHEM 102 Fall 15, LA TECH 14-2-66 Equilibrium Reaction Rates reactions occur faster in gaseous phase than solids and liquids reactions rates increase as temperature increases reactions rates increase as concentration increases rates increase as particle size decreases rates increase with a catalyst CHEM 102 Fall 15, LA TECH 14-2-67