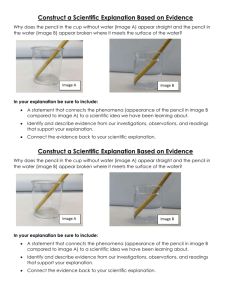
Physical Science Do Now
advertisement

Physical Science Do Now 1/13/11 1. 2. Sharpen pencil and sit in assigned seat Write down 3 things that students do to make a class great and three things teachers do to make a class great →agenda, notebook, paper, pencil Rules/Procedures/Syllabus/Website Demo- Thinking Like A Scientist Rounding Rules HW- 1. Get your Syllabus/Rules & Expectations Signed Practice: Rounding Numbers with Decimals Round correctly to two places after decimal (hundredths) 1. 2. 3. 4. 5. 6. 7. 8. 9. 99.689 4.449 12.642 55.569 222.678 4789.899 3.275 999.474 0.267 Do Now 1/14/11 For each of the devices above: 1. indicate whether it measures distance, time, or speed. 2. indicate which of the following units are possible for Time, distance, or speed: meters (m), seconds (s), or meters per second (m/s). Daily Plan 1/18/11 →pencil, paper • • Chapter 11 Super Summary: Motion Speed Problems (next slide) Do Now 1/19/11 Please do the following metric conversions: a. 397.54 m to mm b. 86 cm to km c. 20 mg to kg d. 2 L to mL →pencil, paper, HW Metric Conversions #1-16, Chapter 11 Super Summary, calculator • Go over HW Metric Conversions #1-20 • Go over Ch 11 Super Summary • Mini-Lesson: Scientific Variables for Motion • Mini-Lesson & Practice: Calculating Average Speed • Mini-Lesson & Practice: Mixed Problems Copy and Complete! Understanding Scientific Variables Variable Variable name symbol Distance Time Speed Velocity Acceleration SI Unit name SI Unit symbol Speed Problems! 1. 2. 3. 4. 5. 6. speed = ? distance = 200 miles, time = 31 hours speed = ? distance = 45 meters, time = 17 seconds speed = ? distance = 100 meters, time = 247 seconds What is the average speed of a car that travels 300 miles in 4.75 hours? What is the average speed of a snail that crawls 18 centimeters in 45 seconds? If kid on a bicycle travels 14 miles in 1.7 hours, how fast did they ride? 1/20/11 1. If it is 800 miles from Brunswick to Philadelphia and it takes a person 12 hours to make that trip, how fast did they drive, on average? Show all work! 2. Round these to 2 decimal places (nearest hundredth) a) 5.2347 mL b) 23.33579 kg c) 17.797 cm 3. Convert the following SI units: a) 27 L to mL b) 14.97 g to kg c) 12 DL to dL →Formula Sheet, calculator, metric conversions chart Mini-quiz: Rounding Decimals and SI Conversions Speed Lab! Lesson & Practice: Calculating “d” and “t” 1. 2. 1/21/11 How fast is a dog walking, on average, if it goes 35 miles in 7.67 hours? Show all work! Prepare for Notebook quiz! Organize… →All notes& Do-Nows in order by date; calculator; Reference Sheet Review hw/quiz grades Notebook Quiz Speed Lab 1/24/11 1. Grab the packet on the side table. DO NOT WRITE ON THIS PACKET!!!! Grab a ruler. 2. Label your own sheet of paper 1 – 24. Odd numbers on one side and even on the other. See board for example. *Turn in Speed Lab from 1/20 ( if you have not) → Measuring & Conversions ( last practice) Lesson & Practice: Distance-Time Graphs and the stories they tell. 01/25/11 1.Draw this graph. 2. Tell what is happening at each segment on the graph. 3. How do you calculate the speed using this graph? Book Lesson: Acceleration Acceleration Word Problems Concept Review: Acceleration Do Now 1/26/11 A car traveling 50m/s slammed on brakes and came to a stop in 20sec. What was the acceleration? 2. A track star accelerated at the sound of the gun from rest to 8m/s in 0.4sec. What was his acceleration? 3. A speeding car slows down from 85m/s to 65m/s in 10 sec. What is the car’s acceleration? →pencil, HW(back of Accel Prob), calculator • Acceleration Quiz • Mixed Problems (Velocity & Acceleration) • Speed IMAX **Speed/Accel quiz tomorrow** 1. Do Now 1/28/11 Get your book and take out a sheet of paper. 2. Use your book p380 & Define the following types of Friction: a. Static Friction b) Sliding Friction c)Rolling Frict 2. Label the LAB data on pg. 380: “Static, Sliding, & Rolling Friction” →Your book, pencil, paper, calculator. 1. Book-based fill-in notes: Ch 11 Section 3 “Motion & Force” Lab: Static, Sliding & Rolling Friction p. 386 & 387 Video lesson “Episode 401: Newton’s 1st & 2nd Laws of Motion” Do Now 1/28/11 Use your book p380 & Define the following types of Friction: a. Static Friction b) Sliding Friction c)Rolling Frict 2. Take out a sheet of paper. 3. Label: LAB: “Static, Sliding, & Rolling Friction” →Your book, pencil, paper, calculator. 1. Lab Setup: Static, Sliding & Rolling Friction p. 386 & 387 Finish Book-based fill-in notes: Ch 11 Section 3 “Motion & Force” 1. 2. Do Now 2/1/11 If a rocket engine pushes with 9,000 N of force on a 2 kg rocket, how fast will it accelerate? SHOW ALL WORK! F =MA TEST Monday 2/7…start preparing! →Formula Sheet, HW Concept Review sheet (1/31), fill in notes Ch 11 Sec 3 (1/28), paper, calculator Go Over Homework: Concept Review Newton’s 1st & 2nd Laws of Motion Tablecloth Demo- Newton’s 1st Law Motion & Force Concept Review Newton’s First Law Video- No Seat Belt Physical Science Do Now 2/2/11 HAPPY GROUNDHOG DAY! 1. Look at the following illustrations, and identify the forces and motion in each one. 2. In which illustration is it likely that no motion will occur? →Formula sheet, Get calculator Prob from 2/1 Physical Science Daily Plan 2/2/11 Newtonian/Football Clips Problems that use Newtons’ 2nd Law of Motion – go over Pop Quiz Newton’s third law & Demo ***Newton’s 1st & 2nd laws Quiz tomorrow**** Daily Plan 2/3/1 Pick up crossword puzzle and get started. Use all your notebook resources. Get HW out for me to check Do back side of crossword puzzle: Concept review Review do-now & HW Review Newton’s Three Laws NASA & Newton's Laws Quick quiz over Newton’s Laws Gravity lesson & weight calculations Video: Final Frontier Do Now 2/4/11 Are the forces balanced or unbalanced? If unbalanced, what is the net force and how much would the object accelerate? 1. 12 N 2. 40 N 25 kg 45 N 99 N 25 kg 99 N 12 N 3. • • • 8N 25 kg 16 N Notebook quiz Review all quizzes for test Test Review 4. 17 N 25 kg 57 N Do Now 2/7/11 Please get your book, turn to p. 424 and do “Understanding Concepts” #1-4 (just answers) Get study guide out. 1. 2. →Calculator, formula sheet, pencil Test Unit 1 don’t worry, you know this stuff! Ch 3 Matter & Energy Vocab Define: 1. Matter 2. Kinetic Theory 3. Solid 4. Liquid 5. Gas 6. Plasma 7. Fluid 8. Energy 9. temperature 10. thermal energy -Read pp77-81 Section 3-1, #1-6 (Answer w/Complete Sentences) 1. Think about the properties of ice. Ice is somewhat hard and cannot be compressed easily. Which drawing do you think represents a solid? Explain your answer. 2. Think about the properties of gases. They are not hard and they can be compressed. Which drawing represents a gas? Explain your answer. 3. In which state(s) of matter are the particles touching? 4. In which drawing do you think the particles have the least effect on one another? Explain your answer. Physical Science Daily Plan 2/8/11 Complete yesterday’s work in chap 3 Video Clip Go over test “Motion & Forces” (if time) Ch 3 Lecture: Energy and States of Matter Section 1 slides #5-11 Pre-written notes and Questions: States of matter Comparing Temperature Scales Summarizer chart NO SANDALS WEDNESDAY!!! (LAB) Do Now 2/9/11 1. What happens to water molecules when water boils? 2. Melting snow is a change of state from a solid to a what? 3. What is the reverse process of melting? Pencil, clean sheet of paper Check and go over HW “Comparing…Temperature Scales” Game: States of Matter Quick Quiz in notebook Lab Safety Lesson Phase Change Lab NASA video & Phase Changes Summarizer- States of Matter (a.k.a. Phases of Matter) Do Now 2/10/11 Draw & Fill in Table using your notes Solid Liquid Gas Plasma Shape & Volume Behavior of Particles Thermal Energy Examples Summarizer- States of Matter (a.k.a. Phases of Matter) Quiz: States of Matter Triple Point diagram Boyle’s/Charles Law notes & Demo Go over Test Behavior of Gases 1. Think about a fixed quantity of gas, say, 100 trillion molecules of oxygen (O2). If we increased the pressure on it, what would happen to the volume? If we increased the temperature of it, what would happen to the volume? Please copy the two charts below, and complete them with up or down arrows to show increase or decrease, as indicated below. = increase Boyle’s Law Pressure Volume = decrease Charles’ Law Temperature Volume Do Now 2/15/11 If the pressure on a fixed quantity of gas increases, what happens to it’s volume? 2. If the temperature on a fixed quantity of gas increases, what happens to it’s volume? Pencil, paper, calculator 1. Notes- “Heat Transfer: Conduction, Convection, & Radiation” Practice- Calculating Heat Gained or Lost Concept Reviews- “Behavior of Gases” and “Changes of State” Physical Science – DO-NOW 02/16 1. What happens to your hand when you place it above a lighted candle? (Assume you are not touching the flame. Explain the energy transfers on the atomic level. Hint: Remember that warm air rises.) 2. When you sit near a fire, you can feel its warmth on your skin, even if the air is cool. Does this sensation depend upon the fact that warm air rises? If not, what does? Daily Plan 2/16/11 Quick quiz: types of heat transfer Heat gained or lost calculations Get Formula Sheet Concept Review: Energy Transfer Practice calculations in mini quiz Energy transformations notes → paper, pencil, calculator, formula sheet Daily Plan 2/17/11 PICK UP do-now ( KE/PE worksheet) and complete! 3rd block: Review hw concept review Energy transformations notes Energy transformations video clips Energy transformations practice worksheet Energy transformations tree map → paper, pencil, calculator, formula sheet Daily Plan 2/18/11 Energy transformations tree map : See board Go over Energy transformations hw Webquest lab: KE/PE ****2nd block is having a fire drill – be ready! → paper, pencil, calculator, formula sheet Physical Science Do Now 2/22/11 1. A woman puts on her 2.25 gram gold ring. The ring heats up from the room temperature of 20 °C to the room temperature of 34 °C. If the specific heat capacity of gold is 0.13 J/g· °C , how much heat energy did the ring gain? 2. Write which type of heat transfer for each: A. wind B. touching an ice cube C. warming hands beside a fire D. warming hands over a fire 3. Why does the temperature not go up if you add heat energy to boiling water? Physical Science Do Now 2/22/11 : Continued 4. Copy each, then write which specific form and general type of energy each one is: a. food b. a person writing c. electricity flowing d. light e. a noise f. a rock on a cliff g. heat h. a battery i. stored in atomic nuclei Review using mini-boards for quiz Quiz: Heat Transfer & Energy Transfer Future Energy Video: Discovery-United Streaming Intro to Waves—Notes & Videos Wave Demos – Super Slinky Practice with Wave Information 1. 2. Physical Science Do Now 2/23/11 Imagine throwing a rock into a pond or lake. Describe the effect that the rock has on the surface of the water. What happens to a string on a guitar or other stringed instrument when it is plucked? How does this let you hear it? →pencil, paper, mental alertness Turn in HW: 5 ways to conserve energy Intro to Waves—Notes & Videos Wave Demos – Super Slinky Practice with Wave Information PARTS of Wave quiz tomorrow!!! Physical Science Daily Plan 2/24/11 Do # 5-7 on your cryptogram! →paper, pencil, yesterday’s notes Wave Interactions notes Wave Interaction Videos Inquiry Lab: Wave Interactions Notes: Sound Waves Sound Fold Notes 1. Inquiry Lab 2/24/11 Fold your paper into Reflection quarters and label (see example to the right) 2. Using the equipment provided, demonstrate, illustrate, and describe each of the 4 behaviors a.k.a. interactions of waves. Refraction Diffraction Interference Physical Science Daily Plan 2/25/11 Notebook quiz………Get ready!! Dates are 2/4-24 Quiz on parts of wave will be on Monday….it is long! →paper, pencil, yesterday’s notes Notes: Sound Waves Sound Fold Notes Make a song….. Can you hear me? Dixie Cup lab Apollo 13….finish Physical Science Daily Plan 2/28/11 Quiz on parts of wave. Get a piece of paper ready! Do cryptogram # 8-11 →paper, pencil, yesterday’s notes Sound Fold Notes Make a song….. (2nd only) Can you hear me? Dixie Cup lab Physical Science Daily Plan 3/1/11 Do cryptogram # 12-15 →paper, pencil, yesterday’s notes Sound Fold Notes finish (2nd only) – finish dixie cup lab (3rd only) – Tsunami videos Intro. to Light notes Questions on light Electromagnetic spectrum Physical Science Daily Plan 3/2/11 Finish cryptogram # 15- end →paper, pencil, yesterday’s notes Electromagnetic spectrum – finish EM spectrum questions EM worksheet Color ***Test Friday: Energy, Waves, Sound & Light Physical Science Daily Plan 3/3/11 Study cryptogram vocabulary! →paper, pencil, yesterday’s notes Vocabulary activity: counts as Lab grade! Color lesson & demo Color & EM worksheet Review for Test ***Test Friday: Energy, Waves, Sound & Light Physical Science Daily Plan 3/4/11 Pick up “quiz” and do on your own paper!. DO NOT WRITE ON IT! →paper, pencil, yesterday’s notes Review for Test Jeopardy Test Simple Machines ***Test Today: Energy, Waves, Sound & Light Physical Science Do Now 3/7/11 1. Which of the following is an example of work: lifting a brick or carrying a brick? 2. A man pushes against a wall, which doesn’t move. Is this an example of work? 3. A student carries her books to class. Is this an example of work? →pencil, calculator, paper for notes, Formula Sheet Go Over Test on Energy & Waves Lesson/Notes/Calculations: Work! Fold notes: Six Simple Machines Lesson/Notes/Calculations: Mechanical Advantage? FoldNote: Simple Machines Turn to p 438 Make your paper look accordingly 1st class lever 2nd class lever 3rd class lever Pulleys What do I need in my Notes? -Definition -Examples: -Drawing Wheel & Axle Inclined Plane Wedge Screw Do Now 3/8/11 1. Determine if work is being done in the following situations: a. Lifting a spoon to your mouth. b. Holding a large stack of books over your head motionless. c. Letting a pencil fall to the ground 2. How much work is done by a person who uses a horizontal force of 25 N to push a desk 3.0 m? →pencil, paper for quiz, calculator, Machines Cornell notes (from yesterday) EXTRA-CREDIT TURN IN Quick Quiz-Work Lesson/Notes: Simple Machines (foldable) Finish ppt Mechanical Advantage COPY! Do Now 3/9/11 1. When you pry up a nail, you “pay” with ________ in order to gain more________. 2. When you lift up dirt into a truck with a shovel , you “pay” with _______ in order to gain more ________. WORD BANK (use each twice)→ •Force •Distance →pencil, paper Lesson/Practice: Mechanical Advantage of Levers & Inclined Planes, Pulleys Carousel activity: Concentration station! Pulley MA calculation Concept Review Do Now 3/11/11 If a person has to push with 250 Newtons of force to roll a 500 Newton rock up a ramp, what is the mechanical advantage of the ramp? 2. If a person pulls a 5 cm nail with a 50 cm pry bar, what is the mechanical advantage of the pry bar? 3. For each situation, write “work” or “no work”. Pushing a box across the floor against friction Pushing against a wall that does not move Lifting a heavy box Carrying a heavy box Rolling a heavy box on a cart with frictionless wheels 4. Draw each of the 3 classes of levers TEST! 1. Do Now 03/14/11 1. Pick up a copy of “Movement & Forces: Review of Variables & Units” and complete the 1st four rows (Mass, Weight, Length, and Volume) →pencil, calculator, formula sheet Matter inquiry lab: bags of mystery Demonstration: Burning Magnesium Metal Notes: Properties of Matter Foldable: “4-Fold Properties and Changes of Matter” & add vocabulary words Summarizer: Interactive lab Do Now 3/15/11 Get out your copy of “Movement & Forces: Review of Variables & Units” and complete the next four rows (Distance – Acceleration) →pencil, calculator, formula sheet Notes: Properties of Matter Foldable: “4-Fold Properties and Changes of Matter” & add vocabulary words Summarizer: Interactive lab & see next slide for review Quick quiz: properties/changes of matter Intro to density Word problems 1. Do Now cont. 3/15/11 1.. Identify whether the following as Physical Property (PP), Chemical Property (CP), or Physical Change (PC), Chemical Change (CC) A. odor B. melting ice C. luster D. digesting food E. reactivity F. freezing point G. rusting metal Do Now 3/16/11 Get out your copy of “Movement & Forces: Review of Variables & Units” and complete the rest of the worksheet. 2. If 10.0 cm3 of ice has a mass of 9.17 g, what is the density of ice? →pencil, calculator, formula sheet Intro to density Word problems: review Dunkin for Denisty lab Matter videos 1. Do Now 3/17/11 1. 2. What is the density of a piece of wood that measures 3 cm x 4 cm x 5 cm and has a mass of 66 grams? Will it sink or float in water? What is the density of a rock that raises water in a grad. cylinder from 90 ml to 150 ml and has a mass of 56 grams? Will it sink or float in water? Quick quiz: density Intro to matter videos Matter Tree diagram Physical Science Do Now 2/16/11 Write your own definition of energy. 2. Write a short sentence that describes the difference between potential energy and kinetic energy. Pencil, paper Lesson & Practice: Introduction to Energy (KE & PE) Lesson & Practice: Energy Transformations LAB TOMORROW: No sandals…long hair tied back…wear goggles…etc. 1. Daily Plan 8/23/10 Finish Trifold “Measuring Motion” Speed, Distance, & Time calculations Get Formula Sheet Learn the “Egg” Speed Calculations → paper, pencil, calculator, Friday’s tri-fold Physical Science Do Now 8/24/10 →Yesterday’s work “Calculating Distance & Time”, Formula Sheet (Reference Sheet), calculator, pencil Finish Time/Distance/Speed Calculations Lab: “Speed Challenge” Physical Science Do Now 8/25/10 If it is 800 miles from Brunswick to Philadelphia and you can average 65 mi/hr, how long will it take to drive from Brunswick to Philadelphia? Show all work! 2. Take Note: Notebook Quiz Tomorrow! Pretest Friday! →Formula Sheet, calculator, Speed Lab sheet (HW) Lesson: Frames of Reference Lesson & Practice: Distance/Time Graphs Olympic Speed & Acceleration Videos: “Banking on Speed” & “Downhill Science” 1. Physical Science Do Now 8/26/10 Prepare for Notebook Quiz →All notes in order by date; calculator; Reference Sheet, notes from 8/25 (yesterday) “Motion” Notebook Quiz Finish Distance-Time Graph Stories (20 minutes) Lesson & Practice: Acceleration (30 minutes) Big Review: Speed, Distance, Time, & Acceleration Problems (25 minutes) Pretest Tomorrow Quiz Monday- Speed, Distance, Time, & Acceleration Physical Science Do Now 8/27/10 Show all work! Round correctly! →Calculator; Reference Sheet; pencil Physical Science Pre-test Big Review: Speed, Distance, Time, & Acceleration Problems Quiz Monday- Speed, Distance, Time, & Acceleration Physical Science 1. 2. Do Now 8/30/10 A whale has to swim 2000 kilometers from coastal Georgia to waters off the coast of Canada. It can swim 84 kilometers/day. How long will it take to get from Georgia to Canada? An airplane is landing. Its velocity is 40.75 meters/second when its wheels first touch the ground, and it takes 15 seconds to stop after it lands. How fast did it decelerate? → Accel. Word Problems (8/26), Speed, Distance, Time, & Accel. Problems (8/27), Formula Sheet, pencil, calculator Finish Big Review: Speed, Distance, Time, & Acceleration Problems Quiz Speed, Distance, Time, & Acceleration Physical Science Daily Plan 8/31/10 Get YOUR book (=seat number), turn to p. 389, and write brief definition or equation (a.k.a. formula) for each of the 10 Key Terms (in the yellow section). Look on p. 389 for most of them! 15 MINUTES! 2. Pick up a crossword puzzle and begin working on it. →pencil, paper (for lab data collection) Video Notes- Newton’s 1st & 2nd Laws of Motion Friction Lab p. 386-387 1. Physical Science Do Now 9/1/10 1. Look at the following illustrations, and identify the forces and motion in each one. 2. In which illustration is it likely that no motion will occur? →Formula sheet, crossword 8/30, paper Physical Science Daily Plan 9/1/10 “Our” ppt & notes, Newton’s 1st & 2nd laws Table cloth demo Problems that use Newtons’ 2nd Law of Motion Concept Review 1st & 2nd laws (include Orbit lesson for #3) Do Now 9/2/10 1. If a rocket engine pushes with 9,000 N of force on a 2 kg rocket, how fast will it accelerate? SHOW ALL WORK! →Formula Sheet, Concept Review sheet (9/1), Notes Newton’s Laws of Motion (9/1) Go Over Homework: Concept Review Newton’s 1st & 2nd Laws of Motion #3- Quick Lesson on “Orbit & Newton’s Laws” Pop quiz- Calculations using Newton’s 2nd law Penny-in-a-Cup Lab Quick Lesson: Newton’s Third Law of Motion Learn & do Net Force Problems Net Force Problems Are the forces balanced or unbalanced? If unbalanced, what is the net force and how much would the object accelerate? 12 N 2. 45 N 35 N 1. 45 N 45 N 25 kg 25 kg 12 N 3. 18 N 25 kg 12N 4. 1N 25 kg 99 N →Formula Sheet, blank paper, PREPARE for notebook quiz Physical Science Do Now 9/3/10 Are the forces balanced or unbalanced? If unbalanced, what is the net force and how much would the object accelerate? 12 N 2. 99 N 99 N 1. 40 N 45 N 25 kg 25 kg 12 N 3. 8N 25 kg 16 N 4. 17 N 25 kg 57 N Physical Science Daily Plan 9/3/10 Notebook Quiz! Mass, Weight, and Gravity Lesson & Practice Calculating Your Weight on the Sun, Moon, & Different Planets Video ABC News The Final Frontier Part 1: The Race to the Moon Do Now 9/7/10 1. If it is 98 meters from Rm 31 to Rm 48, and you can average 0.98 m/s, how long will it take you to get from Rm 31 to Rm 48? 2. If an armadillo can cover 15.63 km per day, how far can it travel in 3 days? 3. A dolphin is swimming 4.5 m/s. It senses a fish, and 1.35 seconds later it is swimming 10 m/s. What was its rate of acceleration? 4. If a car engine pushes with 15,500 N of force and the car’s mass is 4200 kilograms, how fast will it accelerate? → Formula Sheet, calculator, pencil Daily Plan 9/7/10 Unit 1 Review “Paper practice” Review game- “Shoot the Hoop” 1. Think about the properties of ice. Ice is somewhat hard and cannot be compressed easily. Which drawing do you think represents a solid? Explain your answer. 2. Think about the properties of gases. They are not hard and they can be compressed. Which drawing represents a gas? Explain your answer. 3. In which state(s) of matter are the particles touching? 4. In which drawing do you think the particles have the least effect on one another? Explain your answer. Physical Science Daily Plan 9/9/10 →paper Go over test “Motion & Forces” Ch 3 Lecture: Energy and States of Matter Section 1 slides #5-11 Pre-written notes and Questions: States of matter Comparing Temperature Scales Summarizer chart NO SANDALS FRIDAY!!! (LAB) Do Now 9/10/10 1. What happens to water molecules when water boils? 2. Melting snow is a change of state from a solid to a what? 3. What is the reverse process of melting? Pencil, clean sheet of paper Check and go over HW “Comparing…Temperature Scales” Summarizer- States of Matter (a.k.a. Phases of Matter) Lecture/Notes: Phase Change (Ch3 Sec 2 #4-7) Lab Safety Lesson Phase Change Lab Invisible Steam is pure gas a.k.a. vapor Visible Steam is tiny, suspended droplets of liquid water 1/26/10 1/26/10 1/26/10 Do Now 9/13/10 1. Pick up a copy of the States of Matter word search and your book. 2. Write your book number in a permanent place in your notebook. 3. On a separate sheet of paper, write a definition for each of the word find vocabulary words. Pencil, paper, book States of Matter Vocabulary word search Go over Phase Change lab Phases of Matter: Temperature vs Heat Graph Phases of Mater: Pressure vs Temperature Graph Physical Science Do Now 9/14/10 1. Think about a fixed quantity of gas, say, 100 trillion molecules of oxygen (O2). If we increased the pressure on it, what would happen to the volume? If we increased the temperature of it, what would happen to the volume? Please copy the two charts below, and complete them with up or down arrows to show increase or decrease, as indicated below. = increase Boyle’s Law Pressure Volume = decrease Charles’ Law Temperature Volume Physical Science Daily Plan 9/14/10 → pencil, paper Lesson/Notes: Boyle’s Law and Charles’ Law Vacuum Pump Demonstrations of Boyle’s Law and Charles’ Law Concept Review: Changes of State Concept Review: Behaviors of Gases p.96-101 Do Now 9/15/10 If the pressure on a fixed quantity of gas increases, what happens to it’s volume? 2. If the temperature on a fixed quantity of gas increases, what happens to it’s volume? Pencil, 2 paper, calculator Note: Cheeto Lab Tomorrow! No Sandals. 1. Review & Quiz- States of Matter Lesson/ Notes: 3 Types of Heat Transfer (Ch 14 sec 1 vid) Practice: Heat Transfer/ Demo: Convection Box Lesson: Specific Heat Capacity & Calculations (sec 2 side 13) Practice: Specific Heat Capacity Calculations Physical Science – DO-NOW 09/16 1. What happens to your hand when you place it above a lighted candle? (Assume you are not touching the flame. Explain the energy transfers on the atomic level. Hint: Remember that warm air rises.) 2. When you sit near a fire, you can feel its warmth on your skin, even if the air is cool. Does this sensation depend upon the fact that warm air rises? If not, what does? Physical Science Do Now 9/16/10 1. A woman puts on her 2.25 gram gold ring. The ring heats up from the room temperature of 20 °C to the room temperature of 34 °C. If the specific heat capacity of gold is 0.13 J/g· °C , how much heat energy did the ring gain? 2. Write which type of heat transfer for each: A. wind B. touching an ice cube C. warming hands beside a fire D. warming hands over a fire 3. Why does the temperature not go up if you add heat energy to boiling water? Hint: what is the additional heat energy being used to do? Pencil, paper, calculator Quiz: Heat Transfer Heat Transfer Lab a.k.a. burning the Cheeto of Science Concept Review- Energy Transfer Physical Science Do Now 9/17/10 A 3500 gram concrete block sits in the sun. The block heats up from 25°C to 37 °C. If the specific heat capacity of concrete is 0.88J/g· °C , how much heat energy did the block gain? 2. Prepare for Notebook Quiz! Pencil, calculator, organized notebook, formula sheet Notebook Quiz 2 Concept Reviews: “Energy Transfer” and “Land & Sea Breezes” Video: “Planet Weather-Heat” 1. Physical Science Do Now 9/20/10 Write your own definition of energy. 2. Write a short sentence that describes the difference between potential energy and kinetic energy. Pencil, paper Lesson & Practice: Introduction to Energy Lesson & Practice: Energy Transformations 1. Physical Science Do Now 9/21/10 1. Copy each, then write which specific form and general type of energy each one is: a. food b. a person writing c. electricity flowing d. light e. a noise f. a rock on a cliff g. heat h. a battery i. stored in atomic nuclei 2. Write the energy transformation that occurs when a Cheeto is burned. Pencil, yesterday’s work “Energy Transformations”, “Review & Reinforcement Heat & Phase Changes” from 9/13. Daily Plan 9/21/10 More Practice: Energy Transformations Accord Commercial Energy Scavenger Hunt HOMEWORK: Study for mini-test tomorrow on Heat Energy & Energy Transformations Physical Science Do Now 9/22/10 How can zero degrees Celsius be both the freezing point and melting point of water? 2. Calculate the heat energy gained by a 4.75 gram gold ring that goes from 22 ◦C to 37 ◦C when a woman puts it on her finger. The specific heat capacity of gold is 0.13 J/g · °C Pencil, calculator, “Review & Reinforcement Heat & Phase Changes” from 9/13. 1. Honda Accord Commercial: Energy Transformations Energy Present and Future ppt Mini-test: Heat Energy & Energy Transformations 1. 2. Physical Science Do Now 9/23/10 Imagine throwing a rock into a pond or lake. Describe the effect that the rock has on the surface of the water. What happens to a string on a guitar or other stringed instrument when it is plucked? How does this let you hear it? →pencil, paper, mental alertness Go over yesterday’s test on Energy Intro to Waves—Notes & Videos Wave Demos – Super Slinky Practice with Wave Information 1. Physical Science Do Now 9/24/10 Copy each type of wave and write if it is mechanical or electromagnetic, ABD if it is transverse or longitudinal. a. Ocean wave b. visible light c. slinky wave d. Sound wave →pencil, paper, mental alertness Speed Video- “World’s Fastest Indian” Physical Science Do Now 9/27/10 1. Pick up and complete “Concept Review- Types of Waves (front only for now) →pencil, last week preprinted notes Intro to Waves Wave Interactions – notes & drawings Wave Interactions – questions Other Wave Videos Wave vocabulary: word scramble 1. Physical Science Do Now 9/28/10 Write the correct wave “behavior” for each (see yesterday’s notes & questions) a. two sound waves “cancel each other out” b. a rain storm makes a rainbow c. you hear an echo d. An ocean wave curves around behind an island →pencil, yesterday’s notes “Wave Interactions” Physical Science Daily Plan 9/28/10 →paper, pencil, yesterday’s notes “Wave Interactions” Wave Interaction Videos Notes: Sound Waves Inquiry Lab: Wave Interactions Sound Fold Notes 1. Inquiry Lab 9/27/10 Fold your paper into Reflection quarters and label (see example to the right) 2. Using the equipment provided, demonstrate, illustrate, and describe each of the 4 behaviors a.k.a. interactions of waves. Refraction Diffraction Interference 1. 2. 3. Do Now 2/10/10 Sound must have a medium through which to travel. Through which medium—solid, liquid, or gas—does sound travel the fastest? On a string instrument, such as a guitar or violin, how does one string make different musical notes? Using wave theory, explain how making sound with a wind instrument is essentially the same as making sound with a string instrument. 1. 2. 3. 4. 5. Do Now 9/29/10 All waves carry _____ •electromagnetic _____ waves are pure energy. •transverse Mechanical waves require a ___. •longitudinal Sound waves are _____ waves. •energy Ocean waves are _____ waves. •medium →pencil, Notes “Intro to Waves” from 9/23 & “Sound” from 9/28, blank sheet of paper for quiz Daily Plan 9/29/10 Quick Lesson: The Doppler Effect (Ch 15 Section 2) Quiz- Intro to Waves & Sound Fold Notes: Sound Video: “World’s Fastest Indian” Do Now 9/30/10 1. Does sound travel faster through a medium that is more dense or less dense? Why? →pencil, your book, “Sound Fold Notes” from 9/29 Finish “Sound Fold Notes” Paper Cup lab Name That Sound/Waves Concept Map Straw Kazoo Lab 1. Do Now 10/1/10 Prepare for Notebook Quiz 2. An airplane can be detected by radar. When radio waves strike an airplane, they are reflected back to a detector and the airplane shows up on a radar screen. Explain how stealth airplanes fly through the air without being detected by radar. 3. Radio waves that carry radio station transmissions and gamma rays that destroy cancer cells are both electromagnetic waves. What property makes one wave harmless and the other destructive? Stealth Fighter F-117A Stealth B-2 Bomber Daily Plan 10/1/10 • • • • • Notebook Quiz Tuning forks Musical Pipes Lesson/Notes/Questions “What is Light” (front only) Prisms Physical Science Do Now 10/4/10 1. Write the colors of the visible spectrum. 2. Matching: 1. see through A. opaque 2. light passes B. transparent 3. blocks all light C. translucent →Pencil, paper, questions from Friday Drawing/Notes: The Electromagnetic Spectrum EM Spectrum Questions Color: Fill-in Notes Color Mixer (if time allows) Physical Science Do Now 10/5/10 1. Pick up and write answers in your notebook for “Quiz- Electromagnetic Radiation” SKIP #7 and #8. NOTE- this is an old quiz, it is just for practice, it is not a quiz grade! →Pencil, yesterday’s notes “EM Spectrum” Lesson/Fill-In Notes: “Color” Demo- Color Mixer Test Review….Test tomorrow on Energy & Waves! Physical Science Do Now 10/6/10 1. Finish your test review sheet from yesterday. If you are finished, just take it out. →Pencil, HW (review sheet), formula sheet Review: Energy & Waves (15 min) Test: Energy & Waves (75 min) Physical Science Do Now 10/7/10 1. Which of the following is an example of work: lifting a brick or carrying a brick? 2. A man pushes against a wall, which doesn’t move. Is this an example of work? 3. A student carries her books to class. Is this an example of work? →pencil, calculator, paper for notes, Formula Sheet Go Over Test on Energy & Waves Lesson/Notes/Calculations: Work! Lesson/Notes/Calculations: Mechanical Advantage Do Now 10/8/10 1. Determine if work is being done in the following situations: a. Lifting a spoon to your mouth. b. Holding a large stack of books over your head motionless. c. Letting a pencil fall to the ground 2. How much work is done by a person who uses a horizontal force of 25 N to push a desk 3.0 m? →pencil, paper for quiz, calculator, Machines Cornell notes (from yesterday) Quick Quiz-Work Lesson/Notes: Simple Machines (foldable & invention) COPY! Do Now 10/12/10 1. When you pry up a nail, you “pay” with ________ in order to gain more________. 2. When you lift up dirt into a truck with a shovel , you “pay” with _______ in order to gain more ________. WORD BANK (use each twice)→ •Force •Distance →pencil, paper Practice Identifying Simple Machines The Board of Education (A Huge Lever) Lesson/Practice: Mechanical Advantage of Levers & Inclined Planes Do Now 10/14/10 1. 2. 3. 4. If a person has to push with 250 Newtons of force to roll a 500 Newton rock up a ramp, what is the mechanical advantage of the ramp? If a person pulls a 5 cm nail with a 50 cm pry bar, what is the mechanical advantage of the pry bar? For each situation, write “work” or “no work”. Pushing a box across the floor against friction Pushing against a wall that does not move Lifting a heavy box Carrying a heavy box Rolling a heavy box on a cart with frictionless wheels Draw each of the 3 classes of levers Daily Plan 10/14/10 →pencil, calculator, formula sheet Review/ Test: Work, Mechanical Advantage, Simple Machines Introduction to Matter: Describing Matter Do Now 10/15/10 1. Pick up a copy of “Movement & Forces: Review of Variables & Units” and complete the 1st four rows (Mass, Weight, Length, and Volume) →pencil, calculator, formula sheet Matter inquiry lab: bags of mystery Demonstration: Burning Magnesium Metal Notes: Properties of Matter Foldable: “4-Fold Properties and Changes of Matter” & add vocabulary words Do Now 10/18/10 1. Pick up a copy of “Movement & Forces: Review of Variables & Units” and complete the next four rows (Distance - Acceleration) →pencil, calculator, formula sheet Notes: Properties of Matter Foldable: “4-Fold Properties and Changes of Matter” & add vocabulary words Review MIDTERM TOMORROW!!!!! Do Now 10/19/10 1.. Identify whether the following as Physical Property (PP), Chemical Property (CP), or Physical Change (PC), Chemical Change (CC) A. odor B. melting ice C. luster D. digesting food E. reactivity F. freezing point G. rusting metal FINISH YOUR UNITS REVIEW (force-power) Midterm Review Work, MA & Simple Machine test Do Now 10/20/10 Take out “Movement & Forces: Review of Variables & Units” and do the next four: distance, speed, velocity, and acceleration. →pencil, paper, calculator, formula sheet, Review of Variables & Symbols Go Over “Movement & Forces: Review of Variables & Units” Mini-Lab: Finding Mass, Volume, & Density Lesson & Practice: Density 1. Do Now 10/21/10 Finish “Movement & Forces: Review of Variables & Units” REVIEW for Density quiz 1. →pencil, calculator, formula sheet, Review of Variables & Symbols, yesterday’s density problems Go over density problems Density quick quiz Lab: Dunkin’ for Density Do Now 10/22/10 1. 2. 3. Pick up crossword puzzle and complete. Begin Combinations of Matter lesson/questions : Tree Map Inquiry Video! →pencil, calculator, formula sheet, Review of Variables & Symbols, yesterday’s density problems Do Now 10/25/10 What is the density of a piece of wood that measures 3 cm x 4 cm x 5 cm and has a mass of 66 grams? Will it sink or float in water? 2. What is the density of a rock that raises water in a grad. cylinder from 90 ml to 150 ml and has a mass of 56 grams? Will it sink or float in water? →pencil, notebook, HW (TREE MAP questions) Lesson/Notes/Questions: Combinations of Matter Review HW questions *Understanding Atoms -Introduction to Atomic Structure -Drawing Bohr Models of Atoms 1. Do Now 10/26/10 A. Copy & fill in the blanks: 1. The number of _____ = the atomic number. 2. The number of _____ = the atomic mass – the atomic number. 3. The number of _____= the number of protons. Word Bank: ●neutrons ●electrons ●protons B. Draw two atoms of carbon, one using the “circles & e-” method, and the other using the “P, N, dot method. →pencil, homework, Periodic Table of the Elements Check Homework Do Now 10/27/10 Draw “P, N, dot” Bohr Models of one atom of the elements Neon & Potassium GET READY FOR QUICK QUIZ!!!!!!! →pencil, Periodic Table of the Elements (PTE), Candy atom lab. DO NOT EAT ANY CANDY UNTIL TOLD TO DO SO!!!!!! Do Now 10/28/10 Get out Valence electron sheet. We will go over…… Test tomorrow!!! →pencil, Periodic Table of the Elements (PTE), Matter Tree Map, Matter 4-Fold (Chemical vs Physical), Concept Review from 10/21 Self-Check: Matter 4-Fold (Chemical vs Physical) Discovery Lesson: Valence Electrons & the PTE Study Guide for Matter & Atoms Test Friday Do Now 10/29/10 Draw a Bohr diagram of each of the following atoms, including the element symbol, protons, neutrons & electrons in their proper places, and the number of valence electrons. O Mg →pencil, Periodic Table of the Elements (PTE) Test Matter & Atoms Do Now 11/1/10 1. Finish Chemical Symbol Parade! →pencil, Periodic Table of the Elements, Lesson & Practice: The Periodic Table of the Elements Do Now 11/2/10 1. Ca Name the group number, family name, period number, and number of valence electrons for each of the following elements: S Li Cl Kr →pencil, YOUR BOOK, PTE from yesterday (with notes on it), HW “Interpreting the PTE” Go over homework “Interpreting the periodic Table” Quick Lab, p. 160, “Cost of Metals” (CW grade) Lesson/ Practice “Lewis Dot Structures” Do Now 11/2/10 1. Using only your periodic table, tell how many valence electrons each element has: A. Be B. Si C. Ne D. Br →pencil, Periodic Table of the Elements, Lesson & Practice: The Periodic Table of the Elements continued Cost of Minerals lab Lewis Dot structure 1. 2. 3. Do Now 11/3/10 Find phosphorous on your Periodic Table. How many electron energy levels would be filled and how many valence electrons? What is the smallest piece of an element that is still that element? _____ Write down the Lewis Dot Structure for phosphorous →Pencil, paper, PTE (plain copy), last night’s HW “Lewis Structures/Periodic Table Basics” Daily Plan 11/3/10 • • • • • Lewis Dot Speed Drills Isotopes- notes Isotopes- popcorn reading Isotope drawings/with Stable Isotope Periodic Table Practice- “Isotopes or Different Elements?” Speed Drill! Lewis Structures Practice 1. Na P N Ne Daily Plan 11/4/10 1. Why is the Periodic Table of Elements……… periodic? Write one complete sentence that describes the relationship between the structure of atoms (think about Bohr Models) and how the Periodic Table of the elements is arranged into rows called periods. Isotope quick quiz Half-life lesson ( Listen and Learn) Speed Drill! Lewis Structures Be 2. As 3. Ar 1. Al Mg Kr K Ca C Pb Cl Xe Quick quiz: Isotope (11/4/10) Write different element or isotope and tell what element! 1.Element X has 8 protons and 8 neutrons Element Y has 9 protons and 8 neutrons. 2. Element A has 17 protons and 18 neutrons. Element B has 17 protons and 17neutrons. 3. Element D has an atomic number of 92 with a mass number of 238. Element E has an atomic number of 92 with a mass number of 138. Do Now 11/5/10 1. The fictitious radioactive isotope curilium-40 has a half-life of 25 years. How old is a piece of wood with ¼ of it’s curilium-40 remaining? →pencil, paper, HW: “Radioisotope Dating” Notebook quiz Half-life practice problems (3 sets of problems)go over Computer time: review atomic structure & PTE Notebook check 6: 11/1-5 1. 2. 3. 4. 5. 6. On the do-now 11/2, what is the answer to question #1 for Li? What is the number of valence electrons for Si? Draw the Lewis Dot structure for Si. On the do-now for 11/3, what is the answer to #2? What is the definition for isotope? ( notes given on 11/3? What is the answer to the do-now on 11/4? Do Now 11/8/10 1. The fictitious radioactive isotope cartoonium-98 has a half-life of 40 years. How old is a piece of wood with 1/32 of it’s cartoonium-98 remaining? →pencil Quick Lesson/Notes: “3 Types of Ionizing Radiation” Quick Lesson/Notes: “3 Types of Nuclear Changes” Nuclear Power Plant Diagram Challenge Do Now 11/9/10 EOCT test practice : Write question and correct answer Fission/Fusion notes Finish worksheet # 11-17 3-2-1 Nuclear Radiation Today pg. 344-351 1. 2. 3. 4. 3-2-1 “Nuclear Radiation Today” 11/9/10 Turn to Chapter 10 Section 3 p. 344-351 Scan through this section and choose which page you want to read. READ every word on the page you chose http://www.youtube.com/watch?v=174tQ_S8iY Write a 3-2-1 about what you have read: Q 3- facts that you learned 2- questions that you have after reading 1- thing that surprised you the most We will “popcorn share” your 3-2-1s 11/10/10 Pick up Hiroshima video worksheet Get ready to watch video : sharpen pencils etc. http://www.youtube.com/watch?v=174tQ_S8iYQ Do Now 11/12/10 1. 2. 3. 4. 5. Atoms of the same element with different # of neutrons. The amount of time it takes for ½ of the atoms of a radioactive isotope to decay. What is the difference between Carbon-12 and Carbon-13? What is the same? The half-life of Palladium-100 is 4 days. If you have a sample of Paladium-100 with 1/16 of its atoms remaining, how old is it? Fusion or Fission: A. Powered the first atomic bombs. B. Powers the sun C. Splitting atoms D. Combining atoms Do-now cont. 11/12 Review materials for quiz/test (Quest) Finish Hiroshima video NCIS episode and how it relates (http://www.youtube.com/watch?v=174tQ_S8i YQ) H-bomb images Do Now 11/15/10 Try to figure these out by remembering prior knowledge 1. A homogeneous mixture, with one part dissolved into the other. 2. The substance that dissolves the other one. 3. The substance that is dissolved into the other one. 4. The maximum amount of solute that will dissolve into the solvent. *solute *solvent *solution *solubility Lessons/Practice: Solutions Lesson/Practice Solubility curve Lessons/Practice: pH pH Lab? Do Now 11/16/10 1.Pick up Solubility graph worksheet and complete. 2. Study voc from Basics of Solutions worksheet for quick quiz. Quick quiz Review HW Lessons/Practice: pH pH Lab Quick quiz 11/16/10 1. 2. 3. 4. The maximum amount of solute that will dissolve into the solvent. The substance that dissolves the other one. The substance that is dissolved into the other one. Give an example of a concentrated solution. Do Now 11/17/10 1.Pick up Crossword puzzle and complete ( Voc from Basics of Solutions worksheet) 2. Study voc from pH worksheet for quick quiz. pH Practice Review HW Lessons/Practice/ Demo: Conductivity Do Now 11/18/10 1.Pick up Crossword puzzle and complete ( Voc from Basics of Solutions worksheet) 2. Turn over Crossword & take Quiz- Conductivity of Solutions →pencil, yesterday’s pH lab pH Practice Open-everything “Test”: Atomic Nucleus & Solutions: Silent 30, Partner 30 HW: EOCT Part I pH Practice 1. 2. 3. If the solution has a pH of 14, it is a strong _________________. If the solution has a pH of 0, it is a strong _________________. If the solution has a pH of 7, it is __________________. 11/19/10 Grab book, get out notes and materials. Re-do ( fix missed questions) Nuclear and Solutions Test EOCT Bingo Do Now 11/22/10 For each element, write how many valence electrons it has and what ion will form. Chlorine Oxygen Nitrogen Lithium Beryllium Hydrogen Potassium Sodium Aluminum Carbon Calcium Magnesium Flourine Bromine Ions Lesson/Practice Covalent/Ionic Bond lesson/practice Bingo? Do Now 11/23/10 COPY and fill in, using the word bank: “_______are atoms with positive or negative electric charge because they have gained or lost _______ ; _______ are atoms of the same element with different mass because they have different numbers of ________.” •neutrons •electrons •ions •isotopes Do Now 11/29/10 How many valence electrons does Bismuth have? 2. Draw the Bohr diagram of Chlorine? 3. Count the atoms of each element in these compound: a. H2SO4 b. CO2 c. Ca(OH)2 1. Lesson: Ionic/Covalent Bonding Lesson: Binary Ionic Compounds Do Now 12/2/10 Copy & Tell whether each of the following compounds are held together with covalent bonds or ionic bonds C19H14O5S MgO C6H12O6 CaCl2 NaHCO3 CO2 AgNO3 Al2O3 Lesson: Balance Equations Do Now: Test Review 12/3/10 What compound will form from the following ions? 1. NH4+ + NO3- 2. NH4+ + S2- 3. Na+ + NO3- 4. Be2+ + NO3- 5. + NH4 + 2SO4 6. Hg2 2+ + 2SO4 Test review continued 1. 2. 3. 4. 5. 6. Tell what the ion charge will be for the following: H, Be, Ar, Cs, Si P Test review continued Tell what type of reaction ( synthesis, decomposition, single replacement or double) 1. Cu + 2AgNO3 → Ag + Cu(NO3)2 PbCl2 + Li2SO4 → 2LiCl PbSO4 CaO + H2O → Ca(OH)2 2KClO3 → 2KCl + 3O2 2. 3. 4. Test review continued 1. Write the name of each of these compounds: H2S CaCl2 NaHCO3 HCl 2. Write the formula for each of these compunds Sodium hydroxide Calcium iodide 3 Do “atom counts” for each of the following: Fe2O4 Al2(SO4)3 2Fe2O4 3Al2(SO4)3 ECOT test prep: Do Now 12/6/10 1. 2. 3. 4. 5. Convert 15.7 m to mm Convert 165 ml to liters Convert 437.2 km to centimeters Convert 200 meters to mm Convert 23.6 meters to km →pencil, paper *Go over test results • Electricity lesson/notes/worksheet • EOCT Review ? Do-Now 01/03/11 HAPPY NEW! Semester in Pictures Project (01/03 &01/04) Finals are on Friday 01/7 and Monday 01/7 Attendance counts this week for days toward exemption. Do Now 3/8/10 1. Write down and memorize this extremely important series of numbers: “2-8-8” Take the sheet “Understanding Different Combinations of Matter” (Notes on back) and do the questions on the front. →pencil, Friday’s sheet “Understanding Combinations of Matter *Finish Combinations of Matter *Understanding Atoms -Introduction to Atomic Structure -Drawing Bohr Models of Atoms 2. Do Now 3/10/10 Pick up and complete “Atomic Structure”, FRONT SIDE ONLY (for now). →pencil, Periodic Table of the Elements, Physical Science Reference Sheet (formulas), calculator *Mid-term Benchmark test -Focus, but don’t worry *Candy Bohr Models 1. Do Now 3/11/10 Take out and complete the back side of “Atomic Structure” 2. Take out HW “Building Atoms” on back side of “Candy Models” sheet. →pencil, Periodic Table of the Elements, HW *Lesson/Practice: “Valence Electrons” 1. -YOU MUST WORK ACROSS, NOT DOWN *More Practice: “Atom Basics” *Crossword Puzzle: “Mid-term review” Do Now 3/12/10 Answer this question with a complete sentence: Explain why we start a new row on the periodic table, in terms of the structure of atoms. →pencil, Periodic Table of the Elements *Word Puzzle Activity: Chemical Symbol Parade *Mind Point Quiz Show *Olympic Physics Videos 1. Do Now 3/16/10 Draw a complete Bohr model of each of the following elements, and write how many valence electrons each element has in one atom. Carbon Oxygen Helium Magnesium →pencil, Periodic Table of the Elements, all notes from Matter & Atoms (including “Phys. & Chem. Properties & Changes 4-Fold” & “Density Calcs.”) *Test Tomorrow on Intro to Matter (Phys. & Chem. Properties & Changes; Density; Intro to Atoms) *Individual Review/Study Guide *MindPoint Quiz Game Review Ch 4- Atoms 1. Do Now 3/17/10 1. A rock lowered into a graduated cylinder raises the water level from 135 ml to 150 ml. If the mass of the rock is 16.5 g, what is the density of the rock? Will it sink or float in water? How do you know? →pencil, completed Study Guide, Periodic Table of the Elements (PTE), Formula Sheet, calculator Last Minute Questions Test: Introduction to Matter and Atomic Structure Lesson: Introduction to the Periodic Table of the Elements (PTE) Do Now 3/18/10 1. Ca Name the group number, family name, and period number for each of the following elements: S Li Cl Kr →pencil, PTE from yesterday (with notes on it) Go Over Test- Intro to Matter & Atoms Finish Lesson “Intro to the PTE” Practice: “Interpreting the PTE” and “Using the PTE” Quick Lab, p. 160, “Cost of Metals” (CW grade) Do Now 3/19/10 1. Pick up a practice sheet and do everything but the Lewis Structure for each element. You can do it; figure it out! Prepare for Notebook Quiz →pencil, notebook 2. Notebook Quiz Learn/Practice Lewis Structures Pull it all together with “Periodic Table Basics” Quick Lab p. 160 “Cost of Metals” 1. 2. 3. Do Now 3/22/10 Find phosphorous on your Periodic Table. How many electron energy levels would be filled and how many valence electrons? Smallest piece of an element that is still that element? _____ Write down the Lewis Dot Structure for phosphorous →Pencil, PTE (plain copy) • Lewis Dot speed drills • Isotopes – popcorn reading • Isotope drawings/with Isotope periodic table • Practice- “Isotopes or Different Elements?” Speed Drill! Lewis Structures Practice 1. Na P N Ne Speed Drill! Lewis Structures Be 2. As 3. Ar 1. Al Mg Kr K Ca C Pb Cl Xe Do Now 3/23/10 1. 2. Write the definition of an isotope. If element “Z” has 35 protons and 45 neutrons and element “Y” has 35 protons and 46 neutrons, are these two different elements or two different isotopes of the same element? Explain →pencil, BOOK!, paper, your BEST concentration Half-life drawings & % Half-life graph introduction (radioactive decay) Half-life (detailed) worksheet – chunk & group it! Introduce half-life: quick lab pg. 335 Do Now 3/24/10 1. The fictitious radioactive isotope curilium-40 has a half-life of 25 years. How old is a piece of wood with ¼ of it’s curilium-40 remaining? →pencil, paper, “Radioisotope Dating” Half-life practice problems (3 sets of problems) Quick Lesson/Notes: “3 Types of Ionizing Radiation” Do Now 3/26/10 Take out your notebooks- the quiz is open note! →pencil, All notes from this week Quiz Atomic Nuclei- Isotopes, Half-Life, Radioactivity, Fission, Fusion, and Nuclear Power Video- Hiroshima Do Now 4/8/10 Out for field trip, did EOCT test review out of the book and a crossword puzzle review Do Now 4/9/10 Copy and remember these simple pH facts: 1. 0 is a strong acid, 7 is neutral, 14 is a strong base 2. “base turns blue” Demo/Lesson/Practice “Electrical Conductivity” Demo/Lesson/Practice “pH” pH Lab Do Now 4/12/10 Take out your colored periodic table and all notes/practice sheets about isotopes, radiation (alpha, beta, gamma), nuclear changes (radioactive decay, fission, fusion), half-life, solutions, conductivity, and pH Review Test- Atomic Nuclei and Solutions Do Now 4/13/10 Pick up a copy of “Making Predictions” complete the front…remember that all atoms “want” a full valence electron level! Take out any periodic table that you have. 1. 2. Lesson- Ions Practicing Ions Lesson- What holds matter together? Ionic & Covalent Bonds “Making Predictions” & “Bond Identification” Notes: Number Symbols in Chemistry Do Now 4/14/10 1. Pick up and complete the test review sheet. Do all questions, even though you may not be sure of al of them! Lesson/Practice- “Counting Atoms” Lesson/Notes- “Binary Ionic Compounds” Practice- “Binary Ionic Compounds” Practice- “Putting it All Together- Predicting Ions and Binary Ionic Compounds” Do Now 4/15/10 COPY and fill in, using the word bank: “_______are atoms with positive or negative electric charge because they have gained or lost _______ ; _______ are atoms of the same element with different mass because they have different numbers of ________.” •neutrons •electrons •ions •isotopes Review/Practice Binary Ionic compounds Lesson/Practice- “Polyatomic Ions” “Last Chance to Really Understand Ionic Compounds” Quiz “Ions & Ionic Compounds” Do Now 4/16/10 Do “atom counts” for each of the following: Mg(NO3)2 2Mg(NO3)2 (NH4)2CO3 Notes: Chemical Reactions Practice- 4 Types of Chemical Reactions Lesson/Practice- Balancing Chemical Reactions Do Now 4/19/10 Do “atom counts” for each of the following: Fe2O4 2Fe2O4 Al2(SO4)3 3Al2(SO4)3 →Periodic Table, Polyatomic ion reference sheet Review & Practice Balancing Chemical Equations EOCT/Grad Test Review Do Now 4/20/10 Tell whether each of the following compounds are held together with covalent bonds or ionic bonds C19H14O5S MgO C6H12O6 CaCl2 NaHCO3 CO2 AgNO3 Al2O3 Daily Plan 4/20/10 Review- Chemistry in Motion Test- Chemistry in Motion Vocabulary Preview- Electricity Do Now 4/21/10 Which do you think has more power, a small river flowing very fast or a big river flowing slowly? → Pencil, calculator Demonstration: Current Electricity Generator Lesson/Notes: Introduction to Electricity Tree Map Practice: Electricity Calculations: Ohm’s Law Do Now 4/22/10 Pick up and complete 2 current electricity practice sheets → Pencil, calculator Lesson/Demo: Static Electricity Practice: Static Electricity Practice Quiz: Electricity Quiz? Do Now 4/23/10 1. Copy this chart and list the symbol, the unit, and the symbol for the unit for each of the following: Symbol? Unit? Symbol for the Unit? Voltage Current Resistance 2. If a fan draws 3 amperes of current on a 120 volt circuit, what is the resistance of the fan? Show work! 3. How much 12 volt current will a car stereo draw if the stereo has 1.5 Ohms of resistance? Show work! Daily Plan 4/23/10 Review: Electricity Quiz: Electricity Review for EOCT: USA Testprep in Media Center Do Now 4/26/10 1. Write each set of SI units in order from smallest to biggest and write what property of matter each set measures (volume, mass, or length) A. meter, kilometer, millimeter, centimeter B. milliliter, liter, deciliter, kiloliter C. kilogram, gram, centigram, milligram 2. If goonium-40 has a half life of 15 years, how old is a sample that has 1/8 of its goonium-40 remaining? Daily Plan 4/26/10 Demo- Magnetic Fields Lesson/Notes- Magnetism Practice- Electricity & Magnetism Do Now 4/27/10 1. If a lost dog can cover 50 km per day, how far can it travel in 3 days? Show all work! S = D/T 2. Brainium-98 has a half life of 5000 years. A mammoth bone starts out with 200 atoms of Brainium-98. If it is found 10,000 years later, how many atoms of Brainium-98 will it contain? → Pencil Test review Electromagnet lab USA Testprep review (Media Center) Physical Science Do Now 4/28/10 Are the forces balanced or unbalanced? If unbalanced, what is the net force and how much would the 12 N object accelerate? 2. 45 N 35 N 1. 45 N 45 N 25 kg 25 kg 12 N 3. 18 N 25 kg 12N 4. 1N 25 kg →Formula Sheet, Periodic Table, Calculator 99 N Do Now 4/29/10 1. A car light bulb draws 4 amperes of current. If all cars run on 12 volt electricity, what is the resistance of the light bulb? V=IR 2. Is the light bulb in #1 running on Alternating Current or Direct Current? → Pencil, calculator Review for Chapter Test: Electricity & Magnetism…Shoot the Hoop. Chapter Test: Electricity & Magnetism Look at Iceland volcano pictures Do Now 4/30/10 1. 2. Convert 15.7 m to mm Convert 165 ml to liters Convert 437.2 km to centimeters 4. Convert 200 meters to mm 5. Convert 23.6 meters to km → Pencil 3. Go over Chapter Test: Electricity & Magnetism Plan semester celebration for next Friday USAtestprep in Media Center: Finish “Chemistry-Chemical Reactions & Properties of Matter”, then play USAtestprep games. Do Now 5/3/10 1. Take out your Physical Science Reference sheet (“formula sheet”) and your Periodic Table. Look them over and write down at least 3 topics that you would like to review before the EOCT on Wednesday & Thursday Open Review Phy Sci Land Do Now 5/4/10 Please number a section of your Do Now paper like this: 1. 2. 3. 4. 5. 6. 7a. 7b. 7c. 8. 9. → Pencil, “formula sheet”, Periodic Table, USA Testprep sheet 1. RISE review Open review USA Testprep Do Now 5/5/10 Get a calculator, sit down, and relax → Pencil 1. Physical Science EOCT Part 1 Do Now 5/6/10 Get a calculator, sit down, and relax → Pencil 1. Physical Science EOCT Part 2 Do Now 5/7/10 1. Sit down & relax →Only Positive Thoughts Year end celebration! Daily Plan 09/09/09 Videos: Tsunami, Tacoma Narrows Bridge, Rogue waves Properties of Sound – fold-notes booklet pg. 542 Practice: Name that sound Inquiry lab: pitch: straws – Kazoos *** We need Dixie cups (paper) and fishing line for a lab on Friday *** Physical Science Do Now 8/14/09 1. List the three types of friction that we discussed yesterday 2. Last chance: Prepare your notebook for the notebook quiz! Put all of your papers & assignments in order, by date, from the first day of school. →lab tray, calculator, Friction Lab data sheet, DT Graphs & the Stories They Tell Finish Friction Lab Lecture/Discussion/Cornell Notes: Forces, Friction, & Motion Notebook Quiz Distance/Time Graphs & The Stories They Tell Crossword Puzzle: Motion Physical Science Do Now 8/17/09 1. Do Chapter Review questions # 7-14. p. 390-391 →textbook, calculator Newtonian Demonstrator Cornell Notes/Powerpoint Lecture: Newton’s 3 Laws of Motion Concept Review Sheet Inertia Demonstration 25 Word Summarizer (every word counts… use rewriting!) Physical Science Daily Plan 8/18/09 1. A marble is rolling around in the back of a small toy wagon as the wagon is pulled along the sidewalk. When the wagon is stopped suddenly by a rock under one of the wheels, the marble rolls toward the front of the wagon. Why does the marble keep going when the wagon stops? (Hint: Consider what it takes to change the velocity of the wagon and the marble.) →assigned book, calculator, last night’s HW (Newton’s Laws), Newtons Laws Concept Review (yesterday’s classwork) Review Newton’s 3 Laws of Motion Newton’s Laws concept review Quick Quiz For Understanding Penny cup mini lab pg. 398 Newton’s Laws calculations Net Force problems – group work & come to board Physical Science Do Now 8/19/09 Are the forces balanced or unbalanced? If unbalanced, what is the net force and how much would the 12 N object accelerate? 2. 45 N 35 N 1. 45 N 45 N 25 kg 25 kg 12 N 3. → 18 N 25 kg 12N 4. 1N 25 kg 99 N Physical Science Do Now 8/19/09 →Last Night’s HW: Applied Net Force Problems Applied Net Force Problems Fettuccini Physics Lab Lesson: Gravity 1. Physical Science Do Now 8/20/09 Be in a seat and absolutely silent by the time the bell rings! →Pencil, calculator 15 minute grace period- Fettuccini Physics lab Mini-Lecture: Gravity (no notes) Practice: Calculating weight, given mass and acceleration due to gravity Mini-Lecture: Calculating weight in different places Collaborative pairs: Calculating Weight on Different Planets 1. 2. 3. Physical Science Do Now 8/21/09 If a car goes from 0 mi/hr to 60 mi per hour in 6 seconds, what was the rate of acceleration? If a 50 Newton force pushes on a 5 kg object, what is the rate of acceleration? Get notebook ready for notebook quiz! →Pencil, Calculator, assigned BOOK Notebook Quiz Review! Unit 1 Test—Force & Motion 1. Think about the properties of ice. Ice is somewhat hard and cannot be compressed easily. Which drawing do you think represents a solid? Explain your answer. 2. Think about the properties of gases. They are not hard and they can be compressed. Which drawing represents a gas? Explain your answer. 3. In which state(s) of matter are the particles touching? 4. In which drawing do you think the particles have the least effect on one another? Explain your answer. Physical Science Daily Plan 8/24/09 →Pencil, paper, assigned BOOK Go Over Test Ch 3 Lecture: Energy and States of Matter Pre-written notes and Questions: States of matter Phase change diagram Summarizer p. 76 (finish for homework) Physical Science Daily Plan 8/25/09 What happens to water molecules when water boils? 2. Melting snow is a change of state from a solid to a what? 3. What is the reverse process of melting? 4. Compare the properties of ice, liquid water, and water vapor. Pencil, paper, last night’s HW (comparison table) Lessons, notes, demos: Properties of Gases! 1. Physical Science Do Now 8/26/09 1. What do we call each of the following changes: -solid to liquid -liquid to gas -solid to gas -liquid to solid -gas to liquid Pencil, paper, yesterday’s notes “Properties of Gases”, calculator Review & Demos: Boyle’s and Charles’ Laws Quiz: Phases of Matter and Properties of Gases Lecture/Notes: Temperature Scales Lecture/Practice: Calculating Specific Heat Capacity Physical Science Do Now 8/27/09 1. Compare a cup with 100 ml of water at 99 °C and a tub with 10,000 ml of water at 45 °C. A. B. Which one has a higher average kinetic energy? Which one contains more thermal energy (heat)? Pencil, paper, yesterday’s classwork “Calculating Heat Gained or Lost”, calculator Finish yesterday’s calculations Heat Transfer Mini-lecture & Practice Heat Transfer Application: Sea & Land Breezes Concept Review: Energy Transfer Lab Safety Physical Science – DO-NOW 08/28 2. What happens to your 3. When you sit near a hand when you place it fire, you can feel its above a lighted candle? warmth on your skin, (Assume you are not even if the air is cool. touching the flame. Explain Does this sensation the energy transfers on the depend upon the fact atomic level. Hint: that warm air rises? Remember that warm air Physical Science Do Now 8/31/09 Pick Up do-now in front of room and complete! (Review of Motion) Pencil, calculator, brain, book REVIEW Motion! Motion Misconceptions sheet: Divide and Conquer then present! Review PE/KE through drawing KE/PE Powerpoint Identify PE/KE through real-world application (go outside) 1. Physical Science Do Now 9/1/09 1. A woman puts on her 2.25 gram gold ring. The ring heats up from the room temperature of 23 °C to her body temperature of 37 °C. If the specific heat capacity of gold is 0.13 J/g· °C , how much heat energy did the ring gain? 2. Write which type of heat transfer for each: A. wind C. warming hands beside a fire B. touching an ice cube D. warming hands over a fire 3. Why does the temperature not go up if you add heat energy to boiling water? Hint: what is the additional heat energy being used to do? Pencil, calculator Physical Science Daily Plan 9/1/09 Intro/Review- Types of energy: Potential & Kinetic Powerpoint/notes Types of energy: PE & KE Law of Conservation of Energy Quick Notes: Forms of energy Application: Identifying Types & Forms of Energy (Outside…no whining!) Physical Science Do Now 9/2/09 Take a “Review & Reinforcement” sheet from the front table and complete it. →pencil, all three sheets from yesterday: Energy notes, Recognizing Potential & Kinetic Energy, Energy Transformations Review/re-teach energy types & forms Learn/Practice Energy Transformations Quiz- Energy: Types, Forms, & Transformations Read section 1 in Chap 15 (pgs. 505-513) Do classification chart pg. 504 Physical Science Do Now 9/3/09 1. 2. IF YOUR WERE NOT HERE YESTERDAY, get from the front table and complete “Review & Reinforcement: Kinetic & Potential Energy” (front) Which type and form of energy is a piece of charcoal? →pencil, previous sheets “Energy Transformations” and “Review & Reinforcement: Kinetic & Potential Energy” Review/re-teach energy types & forms Learn/Practice Energy Transformations Quiz- Energy: Types, Forms, & Transformations Demonstration: Intro to Waves 1. 2. Physical Science Do Now 9/4/09 Write out 2 energy transformations that describe how the woofer in a speaker makes “bass”. Draw the following: A. two parallel lines B. two perpendicular lines →pencil, paper Demonstration: Intro to Waves Cornell Notes: Intro to Waves Practice with Waves Waves Classification Table Physical Science Do Now 9/8/09 1. High energy waves have _________ wavelength and _________ frequency. 2. Low energy waves have _________ wavelength and _________ frequency. Word Bank: *long *short *high *low →pencil, paper, book Notebook Quiz Cornell Notes: Behaviors of Waves Application: Behaviors of Waves Inquiry Activity: Behaviors of Waves Physical Science Do Now 9/9/09 In the diagram, A is the distance from a point on one wave to an identical point on the next wave. What might this distance be called? 1. In the diagram, B is the amplitude of a wave. What do you think this is a measure of? 2. Twenty waves pass by a point in a certain amount of time. Would this be a measure of a wave’s speed or frequency? Daily Plan 09/09/09 Videos: Tsunami, Tacoma Narrows Bridge, Rogue waves Properties of Sound – fold-notes booklet pg. 542 Practice: Name that sound Inquiry lab: pitch: straws – Kazoos *** We need Dixie cups (paper) and fishing line for a lab on Friday *** 1. 2. 3. 4. 5. Physical Science Do Now 09/10/09 All waves carry _____. •electromagnetic _____ waves are pure energy. •transverse Mechanical waves require a ___. •longitudinal Sound waves are _____ waves. •energy Ocean waves are _____ waves. •medium →pencil, pre-printed notes “Introduction to Waves”, exercises “Name That Sound” Physical Science Daily Plan 09/10/09 • • • • • • Name That Sound Doppler Effect Demonstration/ppt Doppler Effect Quiz: Waves & Sound Sound Lab- Straw Kazoos (Resonance Frequency) Sound Lab- Musical Pipes (Resonance Frequency) Complete Fold Notes: The Doppler Effect Physical Science Do Now 09/11/09 Please draw how you think the following wave would look like? 1. Loud, high pitched tire squeal 2. An elephant grumbling softly 3. Loud bass notes 4. A mouse squeaking quietly →Pencil, Sound Fold Notes, Book Physical Science Daily Plan 09/11/09 • • • • • Complete SOUND Fold Notes CONCEPT REVIEW HANDOUT—CH 15 NB Quiz #5: 9/08-11 Dixie Cups/ Tuning Forks Crossword Puzzle—WAVES Energy TEST –Wednesday, 9/16 Physical Science Do Now 9/14/09 1. Answer this question in your own words: “What is light?” 2. A prism bends light into the colors of the rainbow, name this type of wave interaction. →Pencil, book, a calm & focused mind, HW “Concept Review- Wave Interactions (front) & Types of Waves” • “What is Light” Handout & Questions • EM Spectrum—Drawing & Questions p556-558 • EM Wave questions • Read “How do Cell Phones Work” p.559 Physical Science Do Now 9/15/09 1. Write the colors of the visible spectrum. You should be able to do this from memory. 2. Matching: 1. see through A. opaque 2. light passes B. transparent 3. blocks all light C. translucent →Pencil, paper Lessons/Activities: Seeing Colors Physical Science Do Now 9/16/09 1. Pick up the “Energy Language” handout & a Bingo card. 2. Pick 24 words & write them on the BINGO template. ****EM SPECTRUM—Pass up →Pencil, Calculator, paper for Bingo chips, STUDY!!!! *BINGO *ENERGY TEST Physical Science Do Now 9/17/09 You may not think of a door as a simple machine, but it is one. The door functions like a lever. Like other levers, when you exert a force on it (an input force a.k.a. effort force), that force is exerted along the entire door Pick up a copy of the (the output force a.k.a. Do Now Questions at resistance force). the front! Physical Science Do Now 9/18/09 1. Determine if work is being done in the following situations: a. Lifting a spoon to your mouth. b. Holding a large stack of books over your head motionless. c. Letting a pencil fall to the ground 2. How much work is done by a person who uses a horizontal force of 25 N to push a desk 3.0 m? →Pencil, calculator, paper, HW “Reflections on Waves & Energy Test” -Review Simple Machines, Calculate MA, ARM model, BOE Physical Science Do Now 9/21/09 Pick up a book, a calculator, and a handout @ the front and begin working on it. →pencil, book, calculator, Friday’s work (calculating mechanical advantage of inclined planes & levers) • Finish/go over MA calcs: inclined planes & levers • Ppt: Wheel & Axle & Pulley • MA calculations with pulleys • Inquiry Activity: Making an Arm Model 1. Do Now 9/22/09 Pick up and staple together “Identifying Simple Machines” & “Concept Review”, with “Identifying Simple Machines” on top 2. Complete these (15 minutes). Get Busy! →pencil • Go Over Do Now (20 min. total) • Discuss Tomorrow’s “Mini-Test” (5 min) • Finish Arm Model (15 min) • Clever Lever Lab (20 Min) • Simple Machines Scavenger Hunt (30) 1. Do Now 09/23/09 1. When you pry up a nail, you “pay” with ________ in order to gain more________. 2. When you lift up dirt into a truck with a shovel , you “pay” with _______ in order to gain more ________. Use each word twice: •force •distance →pencil, Formula Sheet, calculator, all notes & exercises about Work, Simple Machines, & Mechanical Advantage • • • Review- Work, Simple Machines, Mechanical Advantage Test-Work, Simple Machines, Mechanical Advantage Scientific Method- Independent & Dependent Variables: Mythbusters Videos Do Now 09/24/09 1. Take a copy of “Movement & Forces Review…” 2. Complete the first 4 variables (mass, weight, length, volume), working across. →pencil, book • • • • Go Over Test-Work, Simple Machines, Mechanical Advantage Cornell Notes- Chemical Properties, Physical Properties, Chemical Changes, Physical Changes 4-Corner Fold- see p. 657 Review – Properties & Changes in Matter Do Now 09/25/09 1. Get out your Do Now from yesterday, “Movement & Forces Review…” 2. Complete the next 4 variables (distance, speed, velocity, acceleration), working across. →pencil, calculator, formula sheet, 4-corner project: • • • • • Notebook Quiz Add new examples to 4-corner Density Lesson Density Practice Density inquiry Activity Do Now 09/28/09 1. Get out your DO NOW from yesterday, “Movement & Forces Review…” 2. Complete the last 3 variables (force, work, & power), working across. →pencil, calculator, HW Density Calculations # 1-9 • • • • Finish Density Calculations Lesson- Types & Combinations of Matter Practice- Understanding Combinations of Matter Concept Reviews- Classifying Matter & Properties of Matter Do Now 09/29/09 1. Complete both sides of “Physical & Chemical Properties” (15 minutes) 2. memorize this number sequence: “2-8-8” →pencil, HW “Concept Review Classifying Matter/Properties of Matter” • • • • Cornell Notes- Introduction to Atoms Practice- Intro to Atoms Practice Drawing Atoms BRING A BAG OF SKITTLES BY THURSDAY TO BUILD ATOMIC MODELS WITH! Do Now 09/30/09 1. Pick up and complete the crossword puzzle “Atomic Structure” →pencil, HW atom drawings #1-16 (Hydrogen – Sulfur) Quiz-sub-atomic particles Atomic structure exercises Races: atom building Introduce valence electrons • BRING A BAG OF SKITTLES BY TOMORROW TO BUILD ATOMIC MODELS WITH! Do Now 10/01/09 Pick up & complete the handout Atomic Structure 2. Copy & complete this sentence: “________ go ‘up and down’, while __________ go ‘back and forth’.” word bank: •columns •rows →pencil, pencil, periodic table 1. **BAG OF M&Ms or SKITTLES out on your DESK** Lesson & Practice: Valence Electrons “Candy Models of Atomic Structure” LAB **HW: STUDY!!! Matter & Atomic Structure Test TOMORROW, FRIDAY, 10/2/09 Do Now 10/02/09 Draw a complete Bohr model of each of the following elements, and write how many valence electrons each element has in one atom. Carbon Oxygen Helium Magnesium →pencil, periodic table, formula sheet, 4-Corner 1. Review- Mindpoint Quiz Show Test Properties of Matter & Atomic Structure **HW: Get ready to have fun AND stiLl keep learning next week! Do Now 10/05/09 Pick up a copy of the Mid Term Review Puzzle and complete →pencil, periodic table 1. ** Review- Mid-term Benchmark Test Tomorrow Lesson & Practice- The Periodic Table 1. Do Now 10/6/09 Pick up & complete “Why is the Periodoc Table…Periodic?” →pencil, “illustrated” Periodic Table, Study Notes to Review Go over Matter & Atomic Structure Test MidTerm 1. Do Now 10/6/09 For each of the following elements, describe: A. Group # B. Family “name” C. Period # D. Metal, Metalloid, or Nonmetal • Sodium • Silicon • Chlorine • Neon →pencil, “illustrated” Periodic Table, Study Notes to Review Go over Matter & Atomic Structure Test MidTerm Do Now 10/7/09 1. Open your book to p160, “Quick Lab: The Cost of Metals.” Procedure # 1 & 2, Analysis # 1 & 2. 2. Take out a clean sheet of paper, begin working on the activity. Follow the directions. (20 minutes) →pencil, textbook, “illustrated” Periodic Table, “Why is the Periodic Table Periodic?”, & “Valence Electrons” Periodic Table Basics Lewis-Dot Structures 1. Do Now 10/8/09 For each of the following elements, describe: A. Group # B. Family “name” C. Period # D. Metal, Metalloid, or Nonmetal • Sodium • Silicon • Chlorine • Neon →pencil, yesterday’s CW/HW “Periodic Table Basics”, “illustrated” Periodic Table, “Why is the Periodic Table Periodic?”, & “Valence Electrons” Pulling it all together: Atomic Structure, Valence Electrons, & the Periodic Table of the Elements Introduction to Isotopes Standardized Test Prep p. 172 & 173 Do Now 10/13/09 Get and complete “Using the Periodic Table” →pencil, Periodic Table, get from front “Using the Periodic Table” 1. Periodic Table “Quiz” (not a quiz!) Drawing Isotopes: Draw “P, N, dot” Bohr models of all the stable isotopes for Hydrogen through Neon Speed Drill: Lewis Structures Speed Drill! Lewis Structures Na 2. Be 3. As 4. Ar 1. P Al Mg Kr N K Ca C Ne Pb Cl Xe 1. 2. 3. 4. Do Now 10/16/09 kim & audrey Find phosphorous on your Periodic Table. How many electron energy levels would be filled and how many valence electrons? Smallest piece of an element that is still that element? _____ Write down the definition for ISOTOPE. Turn to p335 in your BOOK. →Pencil, Periodic Table of the Elements (PTE) • Modeling Half-Life p335 • Half-Life Notes & Graph Handout • CHUNK-IT-UP! Do Now 10/16/09 1. Take out a fresh sheet of paper & copy the fraction/percent pie charts on the left board, and copy the title and headings on the right board: “Understanding HalfLives” →Pencil, extremely focused brain! • Lesson & Practice: Understanding Radioactive Decay using the concept of Half-Life Do Now 10/19/09 1. 2. Take out your work from Friday: “Radioisotope Dating” and both sheets called “Half-Life Practice” Pick up a copy of the exercises at the front of the rom & complete both sides, skipping #4 and #33 →Pencil, Fridays work (see above) Review Half-Life/Radioisotope Dating Learn Ionizing Radiation Learn Fission vs Fusion Quiz Tomorrow! Do Now 10/20/09 1. 2. 3. 4. 5. Atoms of the same element with different # of neutrons. The amount of time it takes for ½ of the atoms of a radioactive isotope to decay. What is the difference between Carbon-12 and Carbon-13? What is the same? The half-life of Palladium-100 is 4 days. If you have a sample of Paladium-100 with 1/16 of its atoms remaining, how old is it? Fusion or Fission: A. Powered the first atomic bombs. B. Powers the sun C. Splitting atoms D. Combining atoms Daily plan 10/20/09 Nuclear Quiz Ions Formed Do Now 10/21/09 Try to figure these out by remembering prior knowledge 1. A homogeneous mixture, with one part dissolved into the other. 2. The substance that dissolves the other one. 3. The substance that is dissolved into the other one. 4. The maximum amount of solute that will dissolve into the solvent. *solute *solvent *solution *solubility Lessons/Practice: Solutions Lessons/Practice: pH pH Lab Do Now 10/22/09 For each element, write how many valence electrons it has and what ion will form. Chlorine Oxygen Nitrogen Lithium Beryllium Hydrogen Potassium Sodium Aluminum Carbon Calcium Magnesium Flourine Bromine Conductivity of Solutions Solubility More practice with half-life! Do Now 10/23/09 Pick up & Complete “Half-Life Worksheet” skip #1 2. Take out and study the following: 10/8- Isotopes 10/16- Radioisotope Dating & Half-life lessons(3 sheets) 10/19- Ionizing Radiation; Fission vs Fusion; Nuclear Power Plant 10/20- Ions (3 sheets); Quiz “Understanding Atomic Nuclei” 10/21- Basics of Solutions; pH; pH Lab 10/22- Conductivity of Solutions; Solubility Curves 1. Do Now 10/26/09 Take & Complete “Section 1” test review. READ carefully & do the best you can! →pencil, periodic table of the elements Cornell Notes: Why do Atoms form Bonds? Practice: “Making Predictions” & “Bond Identification” Number Symbols in Chemistry Counting Atoms Do Now 10/27/09 Pick up & Complete the “Test Review” Handout. READ carefully & do the best you can! →pencil, periodic table of the elements 1. “Counting Atoms” Practice Notes: Binary Ionic Compounds Binary Ionic Compound Practice Polyatomic Ions? 1. 2. Do Now 10/28/09 Pick up & Complete the “Test Review” Handout. READ carefully & do the best you can! Skip “A” and “C” on the back Take out your HW & Periodic Table →pencil, periodic table of the elements Polyatomic Ions: Lesson & Practice Quiz: Ions & Ionic Compounds Review Nuclear Test Laptop Procedures/ EOCT Review Do Now 10/29/09 What compound will form from the following ions? 1. NH4+ + NO3- 2. NH4+ + S2- 3. Na+ + NO3- 4. Be2+ + NO3- 5. + NH4 + 2SO4 6. Hg2 2+ + 2SO4 Do Now 10/29/09 →pencil, periodic table of the elements Go Over Test on Atomic Nuclei & Solutions Notes- 4 Types of Chemical Reactions Practice- 4 Types of Chemical Reactions Acting Out Chemical Reactions Do Now 10/30/09 Prepare for Notebook Check Quiz. →pencil, organized notebook! Local Attractions: Cumberland Island Quiz: 4 Types of Chemical Reactions Lesson & Practice Balancing Chemical Equations Mythbusters Do Now 11/2/09 Do “atom counts” for each of the following: Fe2O4 2Fe2O4 Al2(SO4)3 3Al2(SO4)3 →pencil, Friday’s work “Balancing Act” Review & Practice Balancing Chemical Equations EOCT/Grad Test Review: Physics Do Now 11/3/09 Pick up & complete handout “Do Now-Balancing Equations” 2. Get HW out “Balancing Equations” (front) & “Identifying & Balancing Equations” (back) →pencil, HW 1. Balancing Equations Practice PASSWORD GAME 1. Do Now 11/4/09 Tell whether each of the following compounds are held together with covalent bonds or ionic bonds C19H14O5S CaCl2 NaHCO3 2. Write the numbers 1-4 on your Do Now paper. →pencil, paper folded 1/3 for Cornell Notes Cornell Notes: Laws of Conservation of Mass & Energy Pre-Lab & Lab: “Chemical Reaction” Post Lab Do Now 11/5/09 1. Write the name of each of these compounds: H2S CaCl2 NaHCO3 HCl 2. Write the formula for each of these compunds Sodium hydroxide Calcium iodide 3. Which ion will form? Na N B S →pencil, “plain” periodic table, polyatomic ion sheet Review Test Do Now 11/5/09 Tell whether each of the following compounds are held together with covalent bonds or ionic bonds C19H14O5S CaCl2 NaHCO3 MgO CO2 Al2O3 C6H12O6 AgNO3 Do Now 11/9/09 Take a tree map from the front table and do as much as possible using your notes from Friday. →pencil, notes from Friday, calculator Continue Electricity Lesson (fill-in notes) Current Electricity Calculations (Ohms Law) Static Electricity using Van der Graff Generator Do Now 11/10/09 Copy this chart and list the symbol, the unit, and the symbol for the unit for each of the following: Symbol? Unit? Symbol for the Unit? Voltage Current Resistance →pencil, calculator • Review & Quiz- Introduction to Electricity • Speed Drills: Electricity • NOVA Video: “Lightning” 1. Do Now 11/12/09 List the 4 phases (a.k.a. states) of matter. 2. Pick up & complete “Insulators & Conductors”, then flip it over and fill in at least 7 of the “Measuring Current Electricity” blanks on the back →pencil, notes from Monday, Tuesdays HW “Electricity” on the back of “Electric Current” 1. • • • • More Static Electricity Demos Notes & Demos: Magnets Electromagnet Lab Do Now 11/13/09 Pick up & complete front side of “Overview: Electricity” →pencil • • • Review electricity Ring Magnets Experience Post Test Review Do Now 11/16/09 Please copy and answer each of the following questions: 1. Whose responsibility is it to be on time and present in class? 2. Whose responsibility is it to complete and turn in homework assignments? 3. Whose responsibility is it to behave and pay attention in class? 4. Whose responsibility is it to keep your notebook organized? 5. Whose responsibility is it to go over your notes each night if you are not doing well on tests? 6. Whose responsibility is it to eat right and get enough sleep? 7. Whose responsibility is it to study the night before a test? →pencil, all notes & exercises on electricity Do Now 11/17/09 1. 2. 3. Pick up a copy of “Electricity and Magnetism Terms Review”, and do #1-8 on the front, then do #6-10 on the back. Finish the rest if you get done early. If a fan draws 3 amperes of current on a 120 volt circuit, what is the resistance of the fan? How much 12 volt current will a car stereo draw if the stereo has 1.5 Ohms of resistance? →pencil, calculator, all notes & exercises on electricity *Review & Test- Electricity & Magnetism *EOCT Practice Do Now 11/18/09 1. USA testprep in mc Do Now 11/19/09 1. Pick up a copy of “Post Test Review…”, and do #10-15 on the back. Finish the rest if you get done early. →pencil, calculator *Review & Post Test *Mythbusters Video Do Now 11/20/09 1. Write these SI units of length in order from smallest to biggest: meter, kilometer, millimeter, centimeter 2. Write these SI units of volume in order from biggest to smallest: milliliter, liter, deciliter, kiloliter 3. Write these SI units of mass in order from smallest to biggest: kilogram, gram, centigram, milligram →pencil *Review of SI (System Internationale) units *Mythbusters Video (for sure!) Do Now 11/23/09 1. 2. 3. 4. 5. Convert 15.7 m to mm Convert 165 ml to liters Convert 437.2 km to centimeters Convert 200 meters to mm Convert 23.6 meters to km →pencil, paper *Go over Post-test results *EOCT Review *Planet Earth Video Do Now 11/24/09 1. Media center USA testprep review →pencil, paper Do Now 11/30/09 1. 2. If goonium-40 has a half life of 15 years, how old is a sample that has 1/8 of its goonium-40 remaining? Pick up, hole punch, and begin working on the 2004 released EOCT practice test. →pencil, calculator *EOCT Review *Test Anxiety Video Do Now 12/1/09 1. 2. Write out the energy transformation that occurs when you light a match. Be sure to include both the general type and specific form of the energy before and after the transformation. Continue to work on EOCT Practice Test →pencil, calculator, yesterday’s EOCT Practice Test AND Test Anxiety Video Notes *Go Over Test Anxiety Video *Go Over EOCT Review #1-43….challenge: GPS or QCC? Do Now 12/2/09 1. Pick up and complete BOTH SIDES of “Standardized Test Review” from the front table. →pencil *Go Over EOCT Review #46-85….challenge: GPS or QCC? *Planet Earth video *TOMORROW: Report directly yo Media Center for USA Testprep review Do Now 12/3/09 1. USA Testprep review in MC Do Now 12/4/09 1. 2. EVERYONE pick up a copy of “How to Prepare For Tests” IF you signed up for a copy yesterday, please write your name on it. →pencil, your answer sheet from yesterday *Go Over How to Prepare For Tests *Phy Sci Land Do Now 12/8/09 → Do Now 12/9/09 Pick up a calculator, take out your pencil. 2. Turn your phone off, put it in your bookbag, and place the bookbag on the side lab bench. 3. Relax. →pencil 1. Physical Science EOCT, Part I Do Now 12/10/09 Pick up a calculator, take out your pencil. 2. Turn your phone off, put it in your bookbag, and place the bookbag on the side lab bench. 3. Relax. →pencil 1. Physical Science EOCT, Part II Do Now 12/11/09 Relax. → personal awareness Physical Science Video “The World’s Fastest Indian” Do Now 12/14/09 Make a commitment to be aware of any negative self-talk you may be doing today → positive outlook on life *Physical Science Video “The World’s Fastest Indian” *Begin review for final exam Do Now 12/15/09 Think quietly to yourself…how many times did you become aware of negative self talk yesterday? → pencil, all notes! *Review for final exam Do Now 12/16/09 Think quietly to yourself…how many times did you become aware of negative self talk yesterday? Was it different from the day before? → pencil, all notes! *Review for final exam, Part II Do Now 12/17/09 Think quietly to yourself…how many times did you become aware of negative self talk yesterday? → pencil *Final Exam *Video- “Up”