Immunotherapy-Templates and Forms DW
advertisement

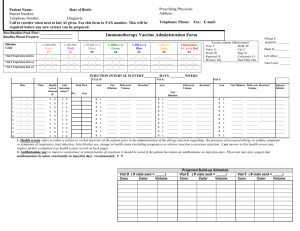
Dana V. Wallace, MD Assistant Clinical Professor Nova Southeastern University Davie, Florida drdanawallace@gmail.com ANAPHYLAXIS IN THE OFFICE ALLERGIST and Staff BE PREPARED Templates and Forms ARE IMPORTANT! Templates & Forms for SIT Cox, L., H. Nelson, et al. "Allergen immunotherapy: a practice parameter third update." J Allergy Clin Immunol 127(1 Suppl): S1-55. – http://www.jacionline.org/article/PIIS009167491001 5034/addons [jacionline] www.acaai.org – (ACAAI > Members > Practice Resources > Skin Testing & Immunotherapy) Kalier, M., Lockey, R., eds. Clinical Allergy and Immunology Series, 4th Edition www.drdanawallace.com Discussing SCIT Treatment Option www.drdanawallace.com SLIT Patient Info (Part 1) SLIT Patient Info (Part 2) SLIT Patient Info (Part 3) SLIT Side Effects Allergy Immunotherapy Consent process should discuss: Treatment and alternatives Potential benefit Potential risks, giving frequency of adverse events, including death Cost associated and coverage options Anticipated duration of Tx Office policies that affect Tx, e.g. waiting time, missed AIs Based on 2011 Immunotherapy PP www.acaai.org Consent to Allergen Immunotherapy CONSENT FORMS TO CONSIDER www.drdanawallace.com Allergy testing & immunotherapy Permission to treat a minor Consent to take allergy vaccine out of office to another MD for administration Consent from remote MD agreeing to administer AI Privacy form to authorize info to specific people- e.g. child custody Consent to take Allergen Extract Sets to another office www.acaai.org Cross-reacting Allergens jacionline Recommended Documentation SCIT Prescription (Rx) Forms jacionline Purpose: – To define the contents of the allergen immunotherapy extract in enough detail that it could be precisely duplicated Patient information: – Name, chart number (if applicable), birth date, telephone number (home/mobile), email, & picture Preparation information: – Name of person (& signature) preparing the allergen immunotherapy extract & date prepared – Vial name, by allergens included (e.g., Trees, Grass or abbreviations (e.g., T, G, with legend) Recommended Documentation SCIT Prescription (Rx) Forms jacionline Allergen immunotherapy extract content information for each allergen: – Common name or genus and species – Concentration of available manufacturer’s extract – Volume of manufacturer’s extract to add to achieve the projected effective concentration • Calculate by dividing the projected effective concentration by the concentration of available manufacturer’s extract times the total volume – Extract manufacturer & lot number, expiration date – Same detail for all mixes Vial expiration date should not exceed of any of the individual components SCIT Prescription Form jacionline SCIT Prescription Form-completed jacionline Name: Jane Doe DOB: Chart #: 3341 Bottle #: Maintenance Concentrate Content Rx Total Vials Allergen, Concentration Available, Manufacturer, Lot # , Expiration date IMMUNOTHERAPY RX FORM MODIFIED BY DANA WALLACE,MD Bottle Name: 8/29/1948 Projected Effective Australian Pine 1:10 1C00061 8/19/03 8/19/03 Australian PineC1:10 C 1C00061 1/1001/100 2 Bald Cypress 1:10 C 2D00061 Bald Cypress 1:10 C1/8/04 2D00061 1/8/04 1/1001/100 3 BayberryBayberry 1:10 C 1J00041 1/8/04 1:10 C 1J00041 1/8/04 1/1001/100 1/10 1/10 0.50 4 Mulberry, Red 1:10 1J44691 3/24/03 3/24/03 Mulberry, Red C1:10 C 1J44691 1/1001/100 1/10 1/10 0.50 5 Baccharia 1:20 G 1:20 225-40-2A19 10/2/03 Baccharia G 225-40-2A19 10/2/03 1/1001/100 1/10 1/10 0.50 6 Dog Fennel 1:10 C 1L3291 Dog Fennel 1:10 C7/14/03 1L3291 7/14/03 1/1001/100 1/10 1/10 0.50 7 Pigweed, Spiny Spiny 1:10 C 1:10 0M00081 8/4/03 Pigweed, C 0M00081 8/4/03 1/1001/100 1/10 1/10 0.50 8 Ragweed, Short Short 1:10 C 1L00191 2/4/03 Ragweed, 1:10 C 1L00191 2/4/03 1/1001/100 1/10 1/10 0.50 9 SheepSheep Sorrel 1:10 C 1G00191 Sorrel 1:10 C 5/1/03 1G00191 5/1/03 1/1001/100 1/10 1/10 0.50 10 Yellow Yellow Dock 1:10 DockC 0M00431 1:10 C 0M00431 7/14/03 7/14/03 1/1001/100 1/10 1/10 0.50 11 None None 0 1/10 1/10 0.00 12 None None 5 5 Anitgen Mite Pteronysinus Mites Farinae Cat Other Animals Standardized Grassed Pollens Molds Projected Effective 0.5 injected 1200 AU/ml 4000 AU/ml 5000 BAU/ml 1:100/ml 8000 BAU/ml 1:100/ml 1:50/ml High Protease:Dust Mites, Molds, Cockroach 4 Concentration Volume in ml Remake if extract to add same Lot available 1/10 1/10 0.50 1/10 1/10 0.50 1 Factor is volume to be injected X 10 A 177 0 1/10 1/10 0.00 A=ALK Total Extract 5.00 C=Center Diluent 0.00 G+Greer Total Volume 55 HS=Hollister Steer Concetration 1ml injected 600AU/ml 2000 AU/ml Date and Signature 2500 BAU/ml 1:200/ml 4000 BAU/ml 1:200/ml 1:100/ml Low Protease: Pollens. Cat, Dog Do not mix high with low protease allergens May mix low with low, high with high Ragweed may be mixed in either group. Keep venomous insects separate Remakes Date, initals & Date, initals & Date, initals & Date, initals & Bottle Color/Vial Dilutions Volume:Volume www.acaai.org (ACAAI > Members > Practice Resources > Skin Testing & Immunotherapy) Brown 1:10,000,000 4 wks Peach Pink Silver Green 1:1,000,000 1:100,000 1:10,000 1:1,1000 4wks 4wks 4wks 6wks Blue 1/100 Expiration Expiration Expiration Expiration 6mths Yellow 1/10 6mths Red Full Strength Earliest expiring constituent Jane Doe Vial A Dana V. Wallace MD 2699 Stirling Road Suite B305 Ft. Lauderdale,FL 33312 954-963-5363 fax 963-7099 Revised 9/02 Labels for allergen immunotherapy extracts jacionline Each vial must have appropriate patient identifiers, e.g., name, number, DOB, picture Contents, e.g, T, G, M, Df, D, etc. The dilution from the maintenance concentrate (vol/vol) using color, numbers, letters Expiration date of individual vial Allergy Extract Vial Dilution & Labeling www.acaai.org Allergy Extract Vial Dilution & Labeling www.acaai.org Vial Labels www.acaai.org Weekly Build-up Therapy jacionline Cluster SCIT Schedule jacionline SCIT Rush Immunotherapy Schedule www.acaai.org SLIT Proposed Schedules SCIT Administration Record List info in separate columns – – – – jacionline Date of injection Arm administered Delivered volume in mm Currently on antihistamine (desirable) Projected build-up schedule Description of any reaction (details may appear on separate sheet Peak flow- pre and post SCIT may be included Best Baseline Peak Flow: ___________________ Date ALLERGY INJECTION ADMIN. FORM Time Health screen Anti-histamine abnormal1 taken?2 Vaccine A: vaccine contents* Peak Arm Flow Vial Number or Dilution Delivered Vaccine B: _______________ Reaction 3 Arm Volume Vial Number or Dilution Delivered Reaction Injector Volume Initials 1. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 2. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 3. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 4. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 5. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 6. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 7. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 8. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 9. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 10. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 11. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 12. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 13. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 14. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 15. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 16. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 17. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 18. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 19. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L ________ ________ _________ _______ 20. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L _______ ________ _________ _______ 21. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L _______ ________ _________ _______ 22. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L _______ ________ _________ _______ 23. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L _______ ________ _________ _______ 24. ___/____/____ _______ Y N Y N ___________ R L ________ ________ ____________ R L _______ ________ _________ _______ 1. Health screen refers to either a written or verbal interview of the patient prior to the administration of the allergy injection regarding: the presence of increased allergy or asthma symptoms or symptoms of respiratory tract infection, beta-blocker use, change in health status (including pregnancy) or adverse reaction to previous injection. A yes answer to this health screen may require further evaluation (see health screen record on back page). 2. Antihistamine use: to improve consistency in interpretation of reactions it should be noted if the patient has taken an antihistamine on injection days. Physician may also request that an antihistamines be taken consistently on injection days: recommended: Y N 3. Reaction: refers to either immediate or delayed systemic or local reactions. Local reactions (noted as LR) can be reported in millimeters as the longest diameter of wheal and erythema.. The details of the symptoms and treatment of a systemic reaction (noted as SR) would be recorded elsewhere in the medical record. Guidelines for dose reduction after a systemic reaction on a separate instruction sheet. Injector signature Initials Date to reorder: __/__/__ www.acaai.org (ACAAI > Members > Practice Resources > Skin Testing & Immunotherapy) Vial 5 Vial 4 SCHEDULE Vial 3 Vial 2 Vial 1 SCIT Administration Record www.acaai.org Health Screen Form (Pre SCIT) jacionline Patient identifiers, date, baseline peak flow & BP, if advised to use antihistamines with SCIT Records status of: – Asthma control, consider standardized instrument and Peak Flow pre and post – Beta-blocker use – Pregnancy or other recent health care status, including recent infection or allergy/asthma flare – Previous adverse reaction to SCIT – Consider BP measurement Health Screen Form jacionline Immunotherapy Pre-Injection Questionnaire PRE-INJECTION HEALTH SCREEN Patient Name:____________________________________ Date:____________________ This questionnaire is designed to optimize safety precautions already in place for your allergen immunotherapy injections (allergy shots). Please review and answer the following questions. The nursing staff will review your responses and notify your physician if they have any questions or concerns whether you should receive your injection(s) today. If you are pregnant or have been diagnosed with a new medical condition, please notify the staff. (Please circle the appropriate answer.) 1. Have you had increased asthma symptoms (chest tightness, increased cough, wheezing, or felt short of breath) in the past week? Yes No 2. Have you had increased allergy symptoms (itching eyes or nose, sneezing, runny nose, post-nasal drip, or throat-clearing) in the past week? Yes No 3. Have you had a cold, respiratory tract infection, or flu-like symptoms in the past two weeks? Yes No 4. Did you have any problems (such as increased allergy or asthma symptoms, hives, or generalized itching or redness) within 12 hours of receiving your last injection? Yes No 5. Are you on any new medications? Any new eyedrops? Please specify.___________________ Staff intervention/office visit: ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ www.acaai.org Staff Signature:_________________________________________________________________ Preparing your office staff for ANAPHYLAXIS ANAPHYLAXIS CART Supplies and Equipment for Anaphylaxis Treatment in office “NECESSARY” Stethoscope and sphygmomanometer *Epinephrine 1:1000 Oxygen IV Fluids Tourniquets, syringes, hypodermic needles, large-bore needles * Required 2011 JTF Anaphylaxis PP “CONSIDER HAVING” One-way valve facemask Diphenhydramine inj. Corticosteroids inj. “MAYBE” Vasopressor (Dopamine) Glucagon Automatic defibrillator Oral airway ANAPHYLAXIS CART Algorithm for Tx Twin Jet Nebulizer Treatment Recording Sheet Ambu Bag- Child (450 ml) (2) Adult (1500ml) (2) (Disposable, latex-free) Defibrillator with heart monitor (Optional) ANAPHPYLAXIS CART INVENTORY AND UPDATE LIST IV ADMINISTRATION IV Pole 1000cc .9 Normal Saline (4 bags) Hydroxyethyl starch (Hespan) 500 ml bag (2) 3-Way Stopcock (2) Micro drip IV set (2) 60 gtt/ml Stethoscope Laryngoscopes Adult and Pediatric (optional) DRUGS Epinephrine 1:1000 1cc ampules (10) Epinephrine 1:10,000 10cc pre-mixed syringes (2) (Optional) Epinephrine 1:1000 30 cc multidose vials (1) Benadryl (diphenhydramine) IV 1 ml ampule, 50 mg/ml (2) Benadryl liquid 12.5 mg/ml (4 oz) & 25 mg tablets (10) Zyrtec (Cetirizine) 5mg/ml (4 oz), 5 mg (5) & 10 mg (5) tablets Zantac(ranitidine HCL) IV 2 ml vial, 25 mg/ml (2) Zantac(ranitidine HCL) PO 1 oz, 15 mg/ml & 150 mg tablets Albuterol 2.5 mg unit dose (3) or Xopenex 1.25 mg (3) and .63 mg (3) inhalant solution Atrovent Inhalant solution .083% unit doses (2) Aminophylline IV, 500 mg vial , 50 mg/cc(1) Prednisolone Syrup 15 mg/tsp 4 oz Solu-Medrol 1 ml vial, 40 mg/ml (3) Medrol 4 mg tablets # 20 Macro drip IV (2) 10-15 gtt/ml Extension tubing (2) T-Connector (2) Catheters #22, 20, 18 (2 each) Butterfly Needles #21, #19 (3 each) www.acaai.org Pulse Oximeter Sphygmomanometer & cuffs: child, Adult regular, and oversized Syringes 1cc, 5cc, 10cc, 20cc, 50cc (5 each) Needles # 16, #18, #20, #22 (5 each) Tourniquet (2) Cushioned IV Boards (2) 4x4 cotton sponges (10) 1” tape Synthetic (Transpore) Alcohol Swabs (10) Latex Free Gloves- 1 box M, L Saline 30 cc, 10 cc (5 each) D5W 250 ml (4), 500 ml (1), 30 ml (2) Atropine (ipratropium bromide) 4 ml vial, 0.5 mg/ml (2) Dopamine 10cc vial, 40 mg/ml (2) Glucagon 1 mg vial (3 vials) NaHCO3 50 mEq/50 ml (Optional) (2) Calcijex IV calcium 1 mg/ampule (Optional) Valium IV 10 ml vial (Optional) AIRWAY Face mask-infant, toddler, child, adult Oral Airways-6 cm, 7cm, 8cm, 9 cm, 10cm Endotracheal tubes 3.5, 5,6,7, 8, 9, 10 (optional) Scalpel, disposable (2) OXYGEN DELIVERY O2 E-Tank with wheeled carrier Gas regulator Tank wrench Pediatric oxygen mask Adult oxygen mask Nasal canula Extension tubing Note (#) number of units to order Check and restock monthly and after each use: 2005 Year_______ Month Jan. Feb. March Initial Month April May June Initial Month July Aug. Sept. Practice Name Practice Address Practice Phone Number Initial Month Oct. Nov. Dec. Initial ANAPHYLAXIS TREATMENT www.drdanawallace.com TABLE OF ANAPHYLAXIS DRUGS Patient Name_______________________ www.drdanawallace.com Drug Start Strength 1:1000 1ml=1mg= 1000mcg Auto Injector (Epi-Pen or 1:1000 TwinJect) 1ml=1mg Epi Infusion 1st choice 1:1000 after IM 1ml=1mg Add Final dilution or max Method of Delivery A=adult, C=child 1:1000 IM lateral thigh Max .3 mg C 1:1000 IM lateral thigh 1:250,000=4.0μg/ml Epinephrine Aqueous (Epi) 250 ml D5W I V i n f u s i o n Epi IV (or IO) after 1:1000 9-27 ml Saline 1:10,000 IV #1 slow push 1-3ml=1-3mg Over 3 minutes cardiac arrest, if not Flush T with 10 ml responding to infusion or Tracheal (T) 1:1000 None 1:1000, .3 max C saline A, 5ml C Epi IV after cardiac 1:1000 27-45 ml 1:10,000 IV #2 Over 3 minutes Saline arrest, if not responding 3-5ml=3-5 mg to above Epi IV high dose infusion 1:1000 250 ml D5W 1:250,000=4.0μg/ml after cardiac arrest, if not 1mg= 1 ml responding to above 50 mg/ml IV/IM Or 25 mg PO Max 24 hr A=400 mg IM/IV (Benadryl) IV/IM/PO 12.5 mg/5ml PO tablet/capsule C=300 mg PO Cetirizine (Zyrtec) PO 5 mg/5ml or 5 mg, PO 10 mg tablet Ranitidine HCl (Zantac) 25 mg/ml IV/IM 20 ml D5W for IV over 5 minutes IV/IM/PO 75 mg/5 ml or 150 IV mg tablet PO PO Albuterol 2.5 mg in 3 ml .083% Nebulized Levalbuterol (Xopenex) .63-1.25 mg in 3 Nebulized ml Ipratropium bromide .02% in 2.5 ml vial May add to Nebulized (Atrovent) Albuterol or Xopenex Aminophylline 500 mg/10 ml Add to 100 ml IV over 30 minutes (optional) Saline, micro drip Normal Saline 1000 ml bags 1-2 L needed IV infusion over first 60 in adult minutes I M D i p h e n h y d r a m i n a x 1 μ 0 g / m i n A , V i n f u s i o n 500 ml 40 mg/ml Max 2 mg/kg/24 hr 5 mg/5 ml 4 mg 0.5 mg/ml Glucagon (Side effects= 1 mg/ml = N and V) 1000 μg / m Max 2 mg A Max 1 mg C I l D f I 5 n W f u s o i r o n S u a l i s n e e M a x 1 m g C Child < 12 (C) Dose/kg *** 0.01mg (ml) /kg max 0 .3 mg X wt in KG = Dose Q ≤ 5 min Adult (A) 0.2-0.5 mg (ml) X _____ =___ mg (ml) Q ≤5 min SR, .3 mg JR , 0.15 mg <15 kg .15 mg c 1 X _ o n t i n u a l - μ 4 m l / m i c g m / i r o m n i ( d r 1 o n = 5 - p s . 6 / 2 5 m i rapid 3-5 minutes T: .3-.5ml of 1:1000 10-30 ml c 4 - 2 . n t i n u a l m i μ 0 m c l r o g / / m d m i r o i n n p ( s / . 0 0 . μ 1 g / k g / m i n _ _ _ _ _ n u t e 1 . 0 - 6 0 - 1 5 i n u t _ g _ = _ . 2 μ _ 5 m l g m / i n 0 . u p μ 1 t g o / k 1 g / m μ 0 i g / n a m i n ↑ d X_____ =______ ml X_____ X______ X_______ =______ =______ =______ ml ml ml X _ _ _ _ _ _ _ _ n _ _ μ 1 _ g _ = _ . 2 μ _ 5 m l m g / i n ) X______ X______ =______ =_____ 10 mg PO-A mg mg mg Q 6 hr 50 mg IV/IM 1 mg/kg IV/IM X______ =______ mg 150 mg PO 2.5 mg 1.25 mg 2 mg/kg PO 1.25-2.5 mg 0.63-1.25 mg X______ Q 20 min Q 20 min =______ 1.25-2.5 0.63-1.25 mg mg mg 500 mcg= 1 vial 250-500 mcg = ½-1 vial 250-500 = ½-1 vial mcg 5 mg/kg 5 mg/kg X______ =______ mg 20-30 ml/kg 25% first 10 minutes 30 ml/kg X______ =______ ml IV infusion over first 60 minutes IV push Q 6 hours 500 ml 30 ml/kg X______ =______ ml 1 mg/kg 1 mg/kg X______ =______ mg PO PO Subcut. 25-50 mg 20-40 mg .3-.5 mg 0.5 mg/kg 0.4 mg/kg 0.02 mg X______ X______ X______ =______ =______ =______ mg mg mg 1 2 X = m V o v e r 25-50 mg 0 e _ μ ) .1ml/kg (0.01 mg/ kg ) max 0.3 mg=3ml T: .05-.1 ml/kg 1ml/kg (0.1 mg/kg) max 30 ml = m _ 1 mg /kg or 2 mg /kg 2.5-10 mg PO-C I repeat 1x PRN then q 6 hr 1 5 1 1 10-30 ml o - 0 3-5 minutes C e Hydroxyethyl starch Hespan (2nd choice) Methylprednisolone (Solu-Medrol) Prednisolone (Pediapred) Methylprednisolone Atropine Frequency Q 6 hr Continue, but reduce after BP stable ? repeat X1 in 6 hrs ? repeat X1 in 6 hrs Q 10 min. 5 m i n F o 5 - l 1 l 5 o w μ w g / i m t h i n I u n t f e u s i o n - 5 m g m 0 - a 3 x 0 ) μ g / k g ( 1 m g _ _ _ _ _ _ _ _ _ _ _ g Anaphylaxis Simple TX Plan Treatment of Anaphylaxis in the Physicians Office Assess airway breathing, circulation, and orientation Inject epinephrine, 0.3 mg intramuscularly, in the vastus lateralis (lateral thigh) Activate emergency medical services (call 911 or local rescue squad)[Might delay, depending upon severity of reaction. DW] Place patient in recumbent position and elevate the lower extremities, as tolerated Establish and maintain airway Administer oxygen Establish an intravenous line for venous access and fluid replacement; keep open with normal saline [Might delay, depending upon severity of reaction. DW] Consider administration of nebulized albuterol, 2.5-5 mg in 3 mL of saline; repeat as necessary Consider administration of ancillary medications, such as H1, [H2]antihistamine, [and] or a systemic corticosteroid Modified from Cox, et. al. AAAAI/ACAAI JTF Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol. 2007 Dec;120(6):1373-7. POST AN ANAPHYLAXIS PROTOCOL AND/OR ALGORITHM (in visible location ) Allergen Immunotherapy Systemic Reation/Anaphylaxis Treatment Record Name:________________________ Date________________________________ Date of Birth__________________ Prescribing Physician__________________ Allergens: Tree-Grass-Weed-Mites-Cockroach-Animal Dander-Mold-Hymenoptera Prior systemic rxn:__________ Hx of asthma?_____________ Date/time of injection:_________________ Date/time of rxn:____________________ Dilution (Vial #): ________________ New? Yes No History of the systemic reaction (SR): Immediate measures: __Assess airway, breathing, circulation, and orientation __Epinephrine IM into thigh __Activate EMS (call 911 or local rescue squad) Y/N Time called:______AM/PM __Management algorithm reviewed (as needed) ANAPHYLAXIS TX RECORD Signs & Symptoms: Respiratory: Skin : Eye/Nasal: Shortness of breath Hives Runny nose Wheezing Angioedema Red eyes Cough Generalized itch Congestion Stridor Flushing Sneezing Time Resp. rate/ PEFR Pulse/ O2 Saturation Vascular Other: Hypotension Difficulty swallowing Chest discomfort Abdominal pain, nausea, diarrhea Dizziness Diaphoresis Headache BP Intervention, Medications, Exam Comments Time (AM/PM)/ Condition upon release:_____________________________________________________ Patient instructions:______________________________________________________________________ Follow-up call to patient: Time________ Comments:___________________________________________ Clinical impression: True SR Questionable SR No SR Comments:_________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ www.acaai.org Dosage adjustment?: ____________________________________________________________________ Signatures_______________________________ RN ___________________________________MD/DO WAO Grading System for SCIT Systemic Reactions: GRADE 1- one organ system Cutaneous – Urticaria, generalized pruritus, flushing, or sensation of heat or warmth or – Angioedema (not laryngeal, tongue, or uvula) OR Respiratory – Rhinitis symptoms (e.g., sneezing, rhinorrhea, nasal pruritus and/or nasal congestion or – Throat clearing (itchy throat) or – Cough perceived to originate in the upper airway mot eh lung, larynx, or trachea Or – Conjunctival: erythema, tearing, or pruritus – Other: nausea, metallic taste, or headache 42 WAO Grading System for SCIT Systemic Reactions: GRADE 2 Symptoms/signs of more than one organ system present or Lower respiratory Asthma: cough, wheezing, SOB (e.g. < than 40% PEF or FEV1 , responding to inhaled bronchodilator) or Gastrointestinal Abdominal cramps, vomiting, or diarrhea Or Other: uterine cramps Might include any of the symptoms listed in grade 1 Patients may describe a feeling of doom WAO Grading System for SCIT Systemic Reactions: GRADE 3 Lower respiratory Asthma (e.g. 40% PEF or FEV1 ) or Upper respiratory Laryngeal, uvula, or tongue edema with or without stridor Note: Might include any of the symptoms listed in grade 1 and 2 Patients may describe a feeling of doom WAO Grading System for SCIT Systemic Reactions: GRADE 4 Lower or upper respiratory – Respiratory failure with or without loss of consciousness or Cardiovascular – Hypotension with or without loss of consciousness Note: Might include any of the symptoms listed in grade 1, 2, and 3 Adults may describe a feeling of doom WAO Grading System for SCIT Systemic Reactions: GRADE 5 Death [We Must Prevent] Thank You DANA WALLACE, MD drdanawallace@gmail.com www.drdanawallace.com MEDICALPROFESSIONAL (USER NAME) Allergy (PASSWORD)