Glucose
advertisement

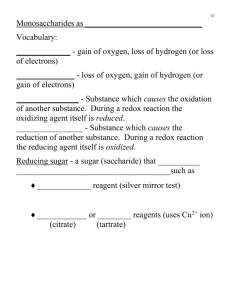
Lecture 5 Carbohydrates and Glycobiology Life Is Sweet Outline •Definitions •Classification •Structure & Function of Carbohydrates 1. Introduction • Carbohydrate, as the name implies, consist of carbon, hydrogen, and oxygen. – Hydrate=(water) hydrogen and oxygen. • The basic formula for carbohydrates is CH2O, meaning that there is one carbon atom, two hydrogen atoms, and one oxygen atom as the ratio in the structure of carbohydrates – What would be the formula for a carbohydrate that has 3 carbons? (1) Where Do Carbohydrates Come From? • Plants take in Carbon dioxide (CO2), water (H2O) and energy from the sun and make glucose– photosynthesis. 6CO2+6H2O+energy (from sunlight) C6H12O6+6O2 (2) Functions • Provide Energy • structural components – cellulose in plants and chitin in arthropods. • Transfer to other life molecules such as lipids and protein • Informational molecules for recognition (3) Nomenclature • Carbohydrates are polyhydroxy aldehydes or ketones, or their derivatives. (4) Classifying Carbohydrates • Scientist use the word saccharides to describe sugars. • Monosaccharides (simple sugers): – only one sugar molecule, cannot be hydrolyzed to simpler sugars, – eg:glucose, fructose and galactose • Oligosaccharides: – 'a few' ( from two to ten monosaccharide units )covalently linked monosaccharides – eg:sucrose, maltose, lactose • polysaccharides: – 'many' covalently linked monosaccharides – eg:Starch, Glycogen, Cellulose (a form of fiber) Glycoconjugates •Also called complex saccharide •Polysaccharide linked to proteins or lipids including •Proteoglycans •Glycoproteins •Glycolipids 2. Monosaccharide (1) Monosacchrides can be divided into two families: aldoses and ketoses. H H C aldehyde group H O C OH C O HO C H H C OH HO C H H C OH H C OH H C OH H C OH CH 2OH CH 2OH D-Glucose, an aldohexose D-Fructose, a ketohexose keto group • Monosaccharides can be classified according to the number of carbon atoms they contain –3 –4 –5 –6 –7 carbons carbons carbons carbons carbons : : : : : triose. tetrose. pentose. hexose. heptose. 3 carbons : Triose • The simplest aldose is glyceraldehyde • The simplest ketose is dihydroxyacetone 5 carbons : Pentose 6 carbons : Hexose •The most abundant monosaccharide in nature is the six-carbon sugar D-glucose. (2) Configuration of monosaccharide •Monosaccharides have asymmetric centers: chiral carbon atom except dihydroxyacetone •Opticity: D(Dextrorotatory) and L(Levorotatory) •Glyceraldehyde is conventionally used as the standard for defining D and L configurations enantiomers H 1 CHO 2 C OH CHO HO C H H C OH H 3 C 4 C OH HO C H H 5 C OH HO C H HO 1 CH2OH 2 C O H CH2OH 6CH2OH D-Glucose L-Glucose mirror plane (an aldose) D- and L-glucose CH2OH C O H C OH H 3 C 4 C OH HO C H H 5 C OH HO C H HO H CH2OH 6CH2OH D-Fructose L-Fructose mirror plane (a ketose) D- and L-fructose Enantiomers: mirror images of each other, D- and L-sugars. • D-Sugars predominate in nature (in living organisms) (e.g., D-ribose, D-glucose, Dgalactose, D-mannose, D-fructose) • Each stereoisomer has a different conventional name, ending with “-ose” suffix • Ketoses are often named by inserting an “ul” into the name of the corresponding aldoses (e.g., aldopentose is named as ribose, the ketopentose is named as ribulose Stereoisomers of D-family of aldose Epimer: Two sugars that differ only by the configuration around one carbon. Stereoisomers of D-family of ketose epimer (3) Cyclization of monosaccharide • Less than 1% of each of the monosaccharides exists in open-chain (acyclic) form (Fischer Projection). • Rather, they are predominantly found in a ring form (Haworth Projection). • The aldehyde or ketone group can react with a hydroxyl group to form a covalent bond. • An aldehyde reacts with a hydroxyl group creates a hemiacetal. • A ketone reacts with a hydroxyl group to form a hemiketal. Conversion of a linear form to a Haworth projection anomeric carbon anomer • -OH group of anomeric carbon (C1) and –CH2OH group (C6) : •On the contrary-α •Same side - β Pyranose form (six-membered ring) D-Glucose Furanose form (five-membered ring) •D-Glucose can cyclize in two ways, forming either furanose or pyranose structures. •In general, the pyranose form is favored over the furanose ring for aldohexose sugars. Glucose: linear form Magic words: right, left, right, right; (top down) Glucose: Ring form Magic words: down, up, down (for carbons 2, 3, and 4) Fructose furanose ring •Fructose also forms pyranose rings. •The pyranose form predominates in fructose free in solution, whereas the furanose form is the major one in most of its derivatives. Notice • Terminology describing sugar structure – Enantiomers • D- and L- – Epimers • differ only by the configuration around one carbon – Anomers • α and β configuration (4) Reducing sugar • Sugars that can reduce Fe3+ or Cu2+ ion are called reducing sugars. The carbonyl group is oxidized to carboxyl group. • Monosaccharides are reducing sugars. • In disaccharides or polysaccharides, the end of a chain with a free anomeric carbon is called the reducing end. brick-red precipitates Fructose is a ketose that changes to aldose in a basic solution. H C C R ketose OH H O H H OH C C R enediol O C OH HC R aldose • a ketone cannot be oxidized directly, a keto sugar can be converted to an aldehyde to migrate the carbonyl to the end of the chain. OH • Benedict's reagent and Fehling's reagent are used to test for the presence of a reducing sugar. • Fehling reagents: copper(II) sulfate (CuSO4)、sodium hydroxide(NaOH)、potassium sodium tartrate • Benedict reagents: CuSO4、sodium carbonate (Na2CO3)、sodium citrate (Na3C6H5O7∙2H2O). •The red precipitate is copper(I) oxide, Cu2O. •These reagents can be used to measure the presence of reducing sugars qualitatively and so can monitor the concentration of blood glucose for diabetes. (5) Glycosides • Glycosides are molecules in which a sugar is bound to a non-carbohydrate moiety, usually a small organic molecule. glycosidic bond Glucose glycone aglycone •Glycosides can be linked by an O-, N-, S- or Cglycosidic bond. (6) The three important dietary monosaccharides – Glucose, which is produced in plants during photosynthesis. – Fructose, which is also produced in plants during photosynthesis and found in fruit juices and honey. – Galactose, which is found in milk. 3. Oligosaccharides • Definition: 'a few' ( from two to ten monosaccharide units )covalently linked monosaccharides • 3 disaccharides – Sucrose = glucose-fructose – Maltose = glucose-glucose – Lactose = glucose-galactose 1). Sucrose •Sucrose is known as common table sugar. •Composed of D-glucoses and D-fructose linked by α-1-β-2 glycosidic bond sugarcane Glc(α1 β2)Fru • The hydrolysis of sucrose, will yield both glucose and fructose. • This chemical reaction is achieved by honeybees which use invertase enzymes. • honey - a mixture of glucose and fructose Sucrose is nonreducing sugar H The anomeric carbon atom for glucose is carbon 1 H H O H H H 1 2 3 4 5 6 C O C O C H C O H C O H C O H C O H C O C H C O H C O H C O H H H The anomeric carbon atom for fructose is carbon 2 H H 1 2 • Since the anomeric carbon is involved in a glycosidic bond, sucrose is classified as a nonreducing sugar. H O H H H 3 4 5 6 H 2). Maltose Maltose syrup •Composed of two D-glucoses linked by α-1, 4 glycosidic bond • reducing sugar Glc(α1 4)Glc 3). Lactose lactose also referred to as milk sugar. • Composed of D-galactose and Dglucose linked by β-1,4 glycosidic bond. • reducing sugar Digestion of lactose •The intestinal villi secrete an enzyme called lactase (β-Dgalactosidase) to digest lactose, and produce glucose and galactose, which can be absorbed. • Lactose intolerance – More than half of the world’s adults are lactose intolerance. – Lactose intolerance is the inability to metabolize lactose, because the lactase is absent in the intestinal system or its availability is lowered. Lactose intolerance • In the absence of lactase, lactose remains uncleaved and passes intact into the colon. • The operons of enteric bacteria quickly switch over to lactose metabolism, and produces copious amounts of gas (a mixture of hydrogen, carbon dioxide, and methane). • This, in turn, may cause a range of abdominal symptoms, including stomach cramps, bloating, and flatulence. • Treatment for this disorder is simple to remove lactose from diet. Two kind disaccharides (1)reducing disaccharide (2) nonreducing disaccharide • Key factor: free aldehyde or ketone group ( a free reducing group) REDUCING SUGARS •When Benedicts test is performed with the disaccharides maltose and sucrose, the following result is obtained: Sucrose is a non-reducing sugar SUCROSE RESULT Maltose is a reducing sugar MALTOSE RESULT 4. Polysaccharides • Homoglycans- homopolysaccharides containing only one type of monosaccharide • Heteroglycans - heteropolysaccharides containing residues of more than one type of monosaccharide • Lengths and compositions of a polysaccharide may vary within a population of these molecules •Polysaccharides may be composed of one, two, or several different monosaccharides, in straight or branched chains of varying length (1). Starch •Starch is a polysaccharide carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. •Function: starch is produced by all green plants as an energy store and is a major food source for humans Amylose Amylopectin •Starch is a mixture of unbranched amylose (α1-4 bonds) and branched amylopectin (α 1-4 and α1-6 branchpoints). Starch structure (2) Glycogen •The glucose storage device in animals •D-Glucoses linked byα-1,4 and α-1,6 glycosidic bonds •Glycogen has the same overall structure as amylopectin but there is significantly more branching in this molecule Glycogen • Glycogen is synthesized and stored mainly in the liver and the muscles. (3). Cellulose •Cellulose is the structural component of the primary cell wall of green plants. •Cellulose is the most common organic compound on Earth. About 33% of all plant matter is cellulose. •Cellulose is a polymer of β-glucose units. Cellulose structure • Cellulose is a straight chain polymer. • Intra- and interchain H-bonding gives strength. hydrogen bonds between parallel chains of beta glucose • • In cellulose, sugar units are joined by beta linkages. The straight chains formed by beta linkages is optimal for structural function. •The multiple hydroxyl groups on the glucose residues form hydrogen bonds, holding the chains firmly together side-by-side and forming microfibrils. Cellulose • Cellulosa can not be digested by mammals due to lack of the enzyme that cleaves β-glycosidic bond. • The functions of dietary fiber – Decrease the absorption of glucose and cholesterol from the intestine, increase the bulk of feces, prevent constipation. (4). Chitin •Chitin is a polysaccharide forming the outer skeleton of arthropods (such as insects, crabs, shrimps, and lobsters). •It is a polymer of N-acetylglucosamine(NAG) in β-1 to 4 glycosidic linkage. • Chitin is the main source of production of chitosan, which is used in a number of applications, such as a wound healing agent, surgical thread, flocculating agent and a delivery vehicle for pharmaceuticals and genes. (5). Testing for polysaccharides: the Iodine Test •When iodine solution is added to a suspension of starch, the iodine molecules pack inside the amylose helix to give a blue-black colour. • All monosaccharides and all disaccharides give negative Iodine Tests. 5. Glycoconjugate (complex saccharide) • Glycoconjugates: carbohydrates covalently linked with other chemical species. Glycoprotein Sugar + protein Proteoglycan Glycoconjugate Glycolipid Sugar + lipid Lipopolysaccharide (LPS) •Glycoconjugates are very important compounds. They are involved in cell-cell interactions, including cell-cell recognition, and cell-matrix interactions. (1). Glycoprotein • Glycoproteins: proteins that contain oligosaccharide chains (glycans) covalently attached to their polypeptide side-chains. • O-Glycosidic and N-glycosidic linkages • Oligosaccharide chains exhibit great variability in sugar sequence and composition. O-Glycosidic and N-glycosidic linkages N-acetylgalactosamine (GalNAc) N-acetylglucosamine (GlcNAc) • Glycoproteins play essential roles in the body. For instance, in the immune system almost all of the key molecules involved in the immune response are glycoproteins. •Glycoprotein in cell membrane (2). Proteoglycan • A special type of glycoprotein with sugar weighing about 95% •On cell surface or Extracellular matrix •Essential components of tissue (particularly connective tissue) structure. (3) Glycolipids • Glycolipids are carbohydrateattached lipids. • Their role is to provide energy and also serve as markers for cellular recognition. Points • Definition, function and classification of carbohydrates • Monosaccharides – – – – Aldoses and ketoses Fischer projections and Haworth structures Reducing sugar Terminology describing sugar structure • Enantiomers,Epimers,Anomers • Oligosaccharides – Sucrose, maltose, lactose • Polysaccharides – Starch, Glycogen, Cellulose – Iodine Test • Glycoconjugate (complex saccharide)