Matter
advertisement

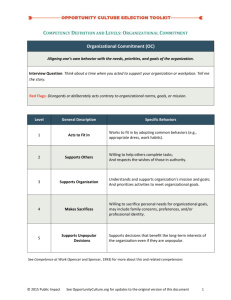
Chem 106, Prof. J.T. Spencer 1 Matter What is Chemistry – Study of the “Physical” Properties Matter (Form and Function) – Study of How Matter Changes (Reactivity) Benefits of Chemistry – Pharmaceuticals – Enhanced food production (fertilizers, herbicides, etc...) – Plastics and Polymers also: environmental BIO economics Why Study Chemistry Medicine electronics – Core requirement (?) Physics agriculture – Central Science politics S.U. CHEM etc... Employment B.S. Engr GEO – Many fields Law Chapt. 1.1 Chem 106, Prof. J.T. Spencer Matter; A Review Definition of Matter – anything that occupies space and has mass States – gas (vapor); no fixed volume or shape, compressable – liquid; fixed volume no fixed shape, mostly incompressable – solid; fixed volume and shape, incompressable Forms – Substances (pure or single); has a fixed composition and distinct properties. Most things encountered are mixtures of substances. Properties – Physical Properties; can be measured without changing the substance, i.e., color, density, melting point, etc... – Chemical Properties; the way a substance changes (reacts), i.e., combustion Chapt. 1.1 2 Chem 106, Prof. J.T. Spencer 3 Matter; A Review Changes – Physical - Changes in appearance but not identity, i.e., evaporation, melting (all changes of state) – Chemical - transformation into a different substance Chemical Changes burning melting C6H12O6 + 6O2 6CO2 + 6H2O chemical reactions NaOH + HCl H2O + NaCl corrosion 4Fe + 3O2 Physical Changes 2 Fe2O3 H2O(s) H2O(l) sublimation H2O(s) H2O(g) dissolution H2O(l ) + NaCl(s) NaCl(aq) Chapt. 1.1 Chem 106, Prof. J.T. Spencer 4 Matter; A Review Mixtures; combinations of substances – Mixture- – Homogeneous – – Heterogeneous - Chapt. 1.1 Chem 106, Prof. J.T. Spencer 5 Matter; A Review Separating Mixtures using Physical Properties – How would you separate; Filtration Sand from Salt Everyday Examples; Auto Oil Filter Auto Air Filter Aquarium Water Filter Spaghetti Strainer Window Screens Registrar Flow Filter Chapt. 1.1 Chem 106, Prof. J.T. Spencer 6 Matter; A Review Separating Mixtures using Physical Properties – How would you separate; Distillation Water from Salt Water NaCl(s) + H2O(l) NaCl(aq) Chapt. 1.1 Chem 106, Prof. J.T. Spencer 7 Matter; A Review Separating Mixtures using Physical Properties – How would you separate; Chromatograpgy Dyes from M&M’s Before After Dyes Chapt. 1.1 Chem 106, Prof. J.T. Spencer 8 Matter; A Review Separating Mixtures using Physical Properties – How would you separate; Salt and Sand Mixture solubility and filtration Ink from Cabbage Juice chromatography Water from Salt Water distillation Iron and Gold Mixture magnetic properties melting point differences chem. reactivity (acids) Iodine from Copper Chloride solubility and filtration Chapt. 1.1 Chem 106, Prof. J.T. Spencer 9 Matter; Elements and Compounds Substances – Elements - substances which cannot be decomposed into simpler substances (see periodic table) – Compounds- substances which can be separated into two or more elements Elements – 110 Known (periodic table to be revisited) – make up all matter and composed of “subatomic particles” – symbols used for abbreviations (from older or common names) Compounds – Elements combined in a definite proportion by mass (law of definite proportion) – properties different than consititutent elements Water; example of mixtures, compound and elements? Chapt. 1.2 Chem 106, Prof. J.T. Spencer 10 Matter Matter No Heterogeneous Mixture Uniform ? Yes Homogeneous No Pure Substance Decomposed ? No Element Yes Compound Can be separated by physical methods Yes Homogeneous Mixture (solution) Chem 106, Prof. J.T. Spencer 11 Matter; Measurement Systems – Metric - base 10 – SI- international scientific system – mass Kilogram – length Meter – time Second – electric current Ampere – temperature Kelvin – light Candela – Amount Mole Factor label method for conversions Chapt. 1.3 Chem 106, Prof. J.T. Spencer 12 Matter; Measurement Prefixes Mega Kilo Deci Centi Milli Micro Nano M k d c m n 106 103 10-1 10-2 10-3 10-6 10-9 Chapt. 1.3 Chem 106, Prof. J.T. Spencer 13 Matter; Measurement Common Units: Length and Mass Length - unit of distance measured in meters Mass - measures the amount of matter in an object in grams Temperature Kelvin Celsius °C = 5/9 (°F -32) Chapt. 1.3 K = °C + 273.15 Chem 106, Prof. J.T. Spencer 14 Matter; Measurement Sample exercise: Ethylene glycol, the major ingredient in antifreeze, freezes at -11.5°C. What is the freezing point in a) K b) °F Chapt. 1.3 Chem 106, Prof. J.T. Spencer 15 Matter; Measurement Derived Units: Volume Length x length x length measured in cm3, which is equal to mL Chapt. 1.3 Chem 106, Prof. J.T. Spencer 16 Matter; Measurement Derived Units: Density amount of mass per unit volume measured in g/cm3, or g/mL Chapt. 1.3 Chem 106, Prof. J.T. Spencer 17 Matter; Measurement Sample exercise: A student needs 15.0 g of ethanol (ethyl alcohol) for an experiment. If the density of the alcohol is 0.789 g/mL, how many milliliters of alcohol are needed? Chapt. 1.3 Chem 106, Prof. J.T. Spencer 18 Matter; Uncertainty in Measurement Precision and Accuracy – Precision - how closely individual measurements agree – Accuracy- how closely the measurements agree with the true value Significant Figures – All measurements are inaccurate intrinsically – measured quantities are reported such that the last figure is uncertain Chapt. 1.4 Chem 106, Prof. J.T. Spencer Matter; Uncertainty in Measurement Good Precision Good Accuracy Good Precision Poor Accuracy Poor Precision Poor Accuracy 19 Chem 106, Prof. J.T. Spencer 20 Matter; Uncertainty in Measurement Determining Significant Figures –all non zero digits are significant –zeros between nonzero digits are significant –zeros to the left of first nonzero digit are not significant –zeros at the end of a number and to the right of a decimal point are significant –when a number ends in a zero but with no decimal point, the zero may or may not be signigicant (use scientific notation) Chapt. 1.4 Chem 106, Prof. J.T. Spencer 21 Matter; Uncertainty in Measurement Determining Significant Figures 3.573 has 4 significant figures 0.073 has 2 significant figures 3.070 has 4 significant figures 0.003 has 1 significant figures - multiplication and division; result can have no more than the figure with the fewest significant figures - addition and subtraction; result can have the same number of decimal places as the term with the least number of decimal places Chapt. 1.4 Chem 106, Prof. J.T. Spencer 22 Matter; Uncertainty in Measurement Sample exercise: How many significant figures are in each of the following measurements? A) 3.549 g B) 2.3 x 104 cm C) 0.00134 m3 Chapt. 1.3 Chem 106, Prof. J.T. Spencer 23 Matter; Uncertainty in Measurement Sample exercise: How many significant figures are in each of the following measurements? A) 3.549 g 4 sig figs B) 2.3 x 104 cm C) 0.00134 m3 Chapt. 1.3 Chem 106, Prof. J.T. Spencer 24 Matter; Uncertainty in Measurement Sample exercise: How many significant figures are in each of the following measurements? A) 3.549 g 4 sig figs B) 2.3 x 104 cm 2 sig figs C) 0.00134 m3 Chapt. 1.3 Chem 106, Prof. J.T. Spencer 25 Matter; Uncertainty in Measurement Sample exercise: How many significant figures are in each of the following measurements? A) 3.549 g 4 sig figs B) 2.3 x 104 cm 2 sig figs C) 0.00134 m3 3 sig figs Chapt. 1.3 Chem 106, Prof. J.T. Spencer 26 Matter; Uncertainty in Measurement Sample exercise: There are exactly 1609.344 m in a mile. How many meters are in a distance of 1.35 mi? Chapt. 1.3 Chem 106, Prof. J.T. Spencer 27 Matter; Uncertainty in Measurement Sample exercise: There are exactly 1609.344 m in a mile. How many meters are in a distance of 1.35 mi? 1.35 mi = 1 mi x 1609.344 m Chapt. 1.3 Chem 106, Prof. J.T. Spencer 28 Matter; Uncertainty in Measurement Sample exercise: There are exactly 1609.344 m in a mile. How many meters are in a distance of 1.35 mi? 1.35 mi = 1 mi x 1609.344 m x = 2172.6144 m Chapt. 1.3 Chem 106, Prof. J.T. Spencer 29 Matter; Uncertainty in Measurement Sample exercise: There are exactly 1609.344 m in a mile. How many meters are in a distance of 1.35 mi? 1.35 mi = 1 mi x 1609.344 m 1.35 has 3 sig figs 1609.344 has 7 sig figs 1 is infinitely significant x = 2172.6144 m Chapt. 1.3 Chem 106, Prof. J.T. Spencer 30 Matter; Uncertainty in Measurement Sample exercise: There are exactly 1609.344 m in a mile. How many meters are in a distance of 1.35 mi? 1.35 mi = 1 mi x 1609.344 m 1.35 has 3 sig figs 1609.344 has 7 sig figs 1 is infinitely significant x = 2172.6144 m x = 2170 m Chapt. 1.3 Chem 106, Prof. J.T. Spencer 31 Dimensional Analysis Use Units throughout the calculation (helps “guide” calculation. Should always yield the proper units Uses conversion factors Example; How fast is 50 mph in in/sec.? 50 mi. 1 hour 1 hour 3600 sec. 5280 ft 1 mi. 12 in. 1 ft = in sec. Chem 106, Prof. J.T. Spencer 32 Dimensional Analysis Sample exercise: By using a conversion factor from the back inside cover, determine the length in kilometers of a 500.0 mi automobile race. Chapt. 1.3 Chem 106, Prof. J.T. Spencer 33 Dimensional Analysis Sample exercise: By using a conversion factor from the back inside cover, determine the length in kilometers of a 500.0 mi automobile race. 500.0 mi Chapt. 1.3 Chem 106, Prof. J.T. Spencer 34 Dimensional Analysis Sample exercise: By using a conversion factor from the back inside cover, determine the length in kilometers of a 500.0 mi automobile race. 500.0 mi 1 km 0.62137 mi Chapt. 1.3 Chem 106, Prof. J.T. Spencer 35 Dimensional Analysis Sample exercise: By using a conversion factor from the back inside cover, determine the length in kilometers of a 500.0 mi automobile race. 500.0 mi 1 km 0.62137 mi = 804.674 km Chapt. 1.3 Chem 106, Prof. J.T. Spencer 36 Dimensional Analysis Sample exercise: By using a conversion factor from the back inside cover, determine the length in kilometers of a 500.0 mi automobile race. 500.0 mi 1 km = 804.674 km 0.62137 mi * answer can only have 4 sig figs; 804.7 km Chapt. 1.3 Chem 106, Prof. J.T. Spencer 37 Chapter One; Review Matter: Chemical and Physical Changes Elements and Compounds Units of Measurement Uncertainty and Significant Figures Precision and Accuracy “Factor Label” Method (Dimensional Analysis)