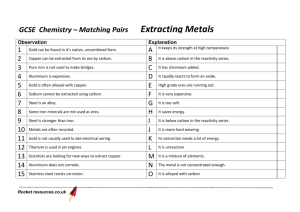
Metals from their ores
advertisement

Metals and their uses Mining for metals What do I need to know Must Discuss the financial benefit and expense of obtaining metals Should Describe how ores and metals are mined Could Explain that reactive metals need a chemical reaction to turn them into the metal What is a metal? Ductile – means it can be pulled into wires Malleable – means it can be bent into shape These are the key properties of metals. What is an ore? • A metal ore is the rock that contains the compound that the metal is found in. • It looks very different to the metal. • It is not usually native metal but a compound such as iron oxide, copper oxide or lead oxide. • The ore has different properties to metals. It does not conduct electricity and cannot be hammered into shape. Mining • Ores are extracted from the earth using mining and quarrying. • This can cause damage to the environment if open quarrying is used and may be noisy and dusty. • There will also be lorries and trucks which produce carbon dioxide as they carry the mined ore away from the quarry or mine. Test your understanding Unreactive metals such as ______ are found in the Earth as the _______ itself. Most metals are mined as ___________ called ores that require chemical reactions to _______ the metal. Metals from ores Reactivity series What do I need to know? Must Recall the position of different elements on the reactivity series Should Describe how the position of an element on the reactivity series determines how to extract it Could Explain that carbon is used in a blast furnace to reduce iron oxide to iron Reactivity series • All metal elements have a different reactivity. • Some such as sodium are very reactive even in air. • Others such as gold are extremely unreactive Recalling the reactivity series More reactive Sodium Magnesium Aluminium Titanium Carbon Zinc Iron Lead Copper Gold Extracting metals • A metal can be extracted from a compound by reacting with an element higher up in the reactivity series. • So for example copper is more reactive than silver. • If we add copper metal to silver nitrate compound we make silver metal. • This is called a DISPLACEMENT REACTION Extracting iron • Iron ore (haematite) is iron oxide. • To make iron we need to remove the oxygen. • This process is called REDUCTION Copy down this equation and the symbol equation. Be able to balance this equation! Iron oxide + carbon Iron + Carbon dioxide 2Fe2O3 + 3C 4Fe + 3CO2 Extracting Iron from its ore • Carbon is part of the reactivity series and is higher (more reactive) than iron. • This means iron can be extracted from its ore using carbon. • This takes place inside a BLAST FURNACE Test your knowledge Metals that are ______ reactive than carbon in the reactivity series can be ___________ from their oxides by _________ with ________. Iron oxide is reduced in the _________ ________ to make iron. [extracted, reduction, carbon, less, blast furnace] Summary Recall the position of different elements on the reactivity series Describe how the position of an element on the reactivity series determines how to extract it Explain that carbon is used in a blast furnace to reduce iron oxide to iron Steelmaking and alloys BOS Process What do I need to know? Recall that most iron is converted into steels. Describe how steels are alloys since they are mixtures of iron with carbon. Explain that alloys can be designed to have properties for specific uses. Why make steel? • Pure iron is relatively soft and not very strong. • The iron from the blast furnace is very hard and brittle. It contains about 4% carbon and is used as cast iron. • We need an iron that is in between these two extremes. Basic oxygen steelmaking Carbon steels can be made by the following processes: • blowing oxygen into molten cast iron to remove most of the carbon as carbon dioxide; then… • adding a calculated amount of carbon. The BOS process Test your knowledge • _____ is made from cast iron by blowing _____ into a large vessel containing molten iron. • Oxygen reacts with _____ in the iron to make carbon dioxide and carbon monoxide • The steel has _____ carbon than cast iron. • ____ _____ is added to cool the reaction. Alloys • An alloy is a metal that contains other elements. • Cast iron is an alloy Alloys with carbon • Low carbon steel is easily shaped (more bendy). • High carbon steel is hard and brittle (strong but not easily shaped). Hardness and tensile strength • Carbon atoms get in the way of the iron atoms making the steel harder and less malleable. • The strength of the steel is also affected by the amount of carbon in the steel. We call this the TENSILE STRENGTH. • It is important to get the right balance between HARDNESS AND TENSILE STRENGTH. Why alloy? To change the properties for example to be: • harder • stronger • rust less or not at all • lighter • heavier • a different colour Stainless steel • This is an example of a very useful alloy of iron. It contains lots of different elements in small amounts. • The most important addition is chromium and this stops the metal from rusting. Other alloys Most metals in everyday use are alloys. are for many uses and so are mixed with small amounts of similar metals to make them harder. Why recycle? You need to know three main reasons: • Preserve raw materials and ores • Save the energy used extracting new metal from its ore • Reduce the waste going to landfill. Extracting reactive metals Reduction of metals high in the reactivity series What do I need to know? Recall that elements below carbon in the reactivity series can be reduced from their ores using carbon in a furnace. Describe how more reactive metals can be obtained using electrolysis. Explain why this means that aluminium and titanium are expensive metals to obtain and how important it is to recycle them. Recalling the reactivity series Sodium Magnesium Aluminium Titanium Carbon Zinc Iron Lead Copper Gold Titanium manufacture • Can you select a metal that could extract titanium from its ore? • In practice magnesium is used. • http://www.youtube.com/watch?v=XsdRo5jv nXo&feature=related • This means that titanium is incredibly expensive due to the many stages required. Test your understanding 1. What is the chemical process called when you obtain a metal from its ore? 2. Which metal is used to extract titanium? 3. Why is titanium so expensive? Titanium Manufacture 1. What is the chemical process called when you obtain a metal from its ore? – Reduction 2. Which metal is used to extract titanium? – Magnesium 3. Why is titanium so expensive? – The process takes a long time, uses expensive raw material, uses a lot of energy and manpower. Aluminium and electrolysis • Aluminium is extracted using • In electrolysis an electric current is used to the aluminium when it is in molten bauxite (aluminium oxide) is added to lower the melting point of bauxite so it can be more easily melted. Aluminium and electrolysis • Aluminium is formed at the negative electrode or Test your knowledge 1. What is the method used to extract aluminium called? 2. What substance is added to help make the aluminium ore (bauxite) molten? 3. Which electrode (positive or negative) does the aluminium form at? 4. Why is making aluminium expensive? Test your knowledge 1. What is the method used to extract aluminium called? REDUCTION BY ELECTROLYSIS 2. What substance is added to help make the aluminium ore (bauxite) molten? CRYOLITE 3. Which electrode (positive or negative) does the aluminium form at? NEGATIVE CATHODE 4. Why is making aluminium expensive? HIGH ENERGY COST TO MAKE ORE MOLTEN. ENERGY USED FOR ELECTROLYSIS Why do we bother? • If electrolysis for aluminium and the extraction of titanium with magnesium are so expensive then why not just use steel? • Aluminium and titanium are valuable metals because of their properties. • Aluminium is light and does not rust • Titanium is hard and has a very high melting point. Copper mining and extraction can be dug up (mined) via traditional means • Copper sulfide ore is turned into copper oxide. This produces sulfur dioxide (SO2) that can contribute to . • Copper oxides is then (burned in a furnace) with carbon. • This to copper in a similar way to the blast furnace for iron, by reacting with carbon to remove the oxygen as CO2. Copper electrolysis • Copper is present in the solution as . • These positively charged copper ions move to the • Here they gain electrons to become Cu or Copper which . • This the copper as other impurities do not travel to the cathode to be deposited. Copper purification Problems with traditional copper mining • Copper is extracted from its ores by chemical processes that involve • this contributes to greenhouse gases and . • copper-rich and • traditional mining and extraction have . Bioleaching • Copper can also come from • Bioleaching is the extraction of copper from its ore through the use of living organisms ( ). • This is much than the traditional leaching using cyanide or smelting as no greenhouse gases are produced from burning coal. Phytomining involves using plants to extract the metal compounds present in . • Once the plants have been grown they are burned to produce . • The metal can then be extracted using . • This is . Summary Recall that elements below carbon in the reactivity series can be reduced from their ores using carbon in a furnace. Describe how more reactive metals can be obtained using electrolysis. Explain why this means that aluminium and titanium are expensive metals to obtain and how important it is to recycle them. Transition metals Uses and properties What do I need to know? Must Recall the general properties of metals and how they differ from non metals Should Describe how the uses of metals are determined by their properties Could Identify where transition metals are found on the periodic table and identify three examples. Transition metals Transition metals are found in the middle section of the periodic table. Find this section on the periodic table in your planner and write down at least three examples of transition metals you have heard of. Two examples are gold, Au and iron, Fe. Some transition metals are very rare and very valuable. Identifying transition metals Circle the elements you think are transition metals. Uses of transition metals • Gold – precious metal, rare so very expensive yellow and shiny, used for jewellery and electrical contacts • Platinum – precious metal, rare so very expensive, silvery, shiny, used for jewellery • Iron – available in large quantities, strong, hard and high tensile strength, can be made corrosion resistant, used in building • Copper – high electrical conductivity, ductile (can be made into wires), malleable (can be made into pipes), used in electrical wires and plumbing. Has coloured compounds. • Zinc – corrosion resistant when galvanised or oxidised, can be given an attractive finish, used in dustbins, wheelbarrows and other outdoor applications. • Tungsten – very high melting point, ductile and high tensile strength, used in lightbulb filaments. Complete the table The first two have been done for you Property Use Example metal Good conductor of electricity Used to make electrical wires Copper Shiny Used to make mirrors and jewellery Silver Good conductor of heat Dense Strong High melting point Magnetic Test your understanding Copper is used to make the electrical wires in our houses. Silver is an even better conductor of electricity than copper. Why don’t we use silver to make the wires for the electric circuits in our houses?