Atomic Charge
advertisement

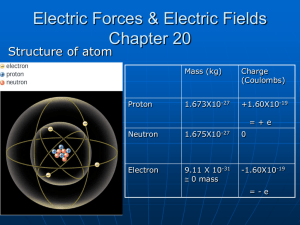
Electrostatics The study of electric charges Introduction • Did you ever run a comb through your hair? What do you notice. • What causes the paper holes to jump onto the comb? • There are electrical forces that are in place due to the presence of charge on the comb. The Atom • An atom consists of various charged and uncharged particles. • The central region is called the nucleus. • Protons (+) and neutrons make up the nucleus. • Electrons (-) move around the nucleus in an orbital path. Nucleus Neutrons Protons (+) Electrons (-) The Significance of Charge • An atom with balanced charges is considered neutral. • The overall charge can be changed by adding or removing electrons. This makes the atom an ion. Add e- Take eOverall Charge: 1 01 (Negative (Neutral Ion) (PositiveAtom) Ion) Sample Problem (Atomic Charge) • • • • -19 An helium atom has a net electric charge of -8.0x10 C. Is it neutral or an ion? Are there extra electrons or a shortage of them? How many extra electrons are there? – Charge Per e-: -1.60*10-19C 19 8.0 10 C #e 1.6 1019 C qNet #e qe #e 5 Sample Problem (Atomic Charge) • • • • -19 An atom has a net electric charge of 4.8x10 C. Are there extra electrons or a shortage of them? How many electrons short is this atom? Draw this atom given it is Boron. Electrostatic Demo’s • Tape • Electroscope Electric Forces • Charges exert a force on other charges Like Charges Repel Opposites Attract Actual Charge of Protons/Electrons • Recall, charge is measured in Coulombs (C). • Even though protons and electrons are very small, they still have charge. • Let us use q as a variable for charge. Electron qelectron 1.60 1019 C Proton q proton 1.60 1019 C How do atoms get charged? • Work can remove electrons from the atom. – Results in a positively charged atom • The free Electron can be transferred to another atom. – Results in negatively charged atoms Separating Charge • Charges are balanced in neutral objects. • Work must be done to separate charge (free electrons). • Once charge is separated, it can be used in experiments. Separation of Charge • Bring a charge rod near a neutral conductor • Un-like charges are attracted • Like charges are repelled Charge by conduction • A charge rod touches a neutral conductor • Like charges are repelled and uniformly distribute Charge by Induction The charges oncharge the spheres redistribute to A Separation charge object of isthe placed takes near place neutral conductors charge object is removed Contact between conducting sphere is maximum broken Result: Two spheres charged by induction separation A B B Charging by Polarization • Certain substances, such as the one below, have polar molecules. These molecules have opposite charges at each end. • Charging by polarization takes place when a charged object is brought near, realigning the molecules in the substance. Magnification Conductors and Insulators • Electrical Conductors are similar to Heat conductors. • Electrical Conductors allow charge to move easily. • Electrical Insulators do not allow charge to move easily Conductors and Insulators • Electrical Conductors all electrons to move easily. – Metals – Graphite • Electrical Insulators do not allow electrons to move easily – Glass – Plastic – Rubber Coulomb’s Law • The electrostatic force one charged object exerts on an other • The force is related to the amount of charge – i.e more charge – more force • The force is proportional to 1/d2 – i.e. the further apart the charges, the smaller the force Coulomb’s Law F Symbol F q1 q2 d K Kq1 q 2 Force Charge Charge Distance constant d2 Unit N C C m N m2 / C2 Electro-static Applications Electrostatic filter Coulomb’s Law in 2-D • • • • To find Fnet with 3 or more charges Calculate each Force vector. It helps to have a grid system on which to work. Use vector addition to find the resultant Fnet q2 q3 F13 q1 F14 F12 FNet q4 Coulomb’s Law in 2-D (cont.) kq1q2 F12 d12 2 Charge (C) q1 3.0 X 10-4 q2 -2.6 X 10-5 q3 7.2 X 10-6 F12 9.0 10 9 Nm2 C2 4 5 3.0 10 C 2.6 10 C 13m 2 F12 5.4 N q2 F12 20 q3 13 F13 q1 kq1q3 F13 d132 F13 9.0 109 Nm2 C2 3.0 10 C 7.2 10 C 4 20m 6 2 F13 0.972 N Coulomb’s Law in 2-D Sample • Determine the direction of each of the forces prior to vector addition. opp tan adj F12 2 tan 3 2 tan 4 33.7 26.6 Quad II Adjust Quad III Adjust 33.7 180 26.6 180 146.3 206.6 1 opp q2 5.4N hyp adj 0.972N hyp q3 F13 1 opp q1 adj Coulomb’s Law in 2-D Sample • The remaining task is to use analytical vector addition. Mag Ang X Y Q F12 5.4N 146.3° -4.49 3.00 II F13 0.972N 206.6° -0.87 -0.44 III FNet 5.94N 154.5° -5.36 II F12 x F12 cos F12 x 5.4 N cos 146.3 F12 x 4.49 N F12 y F12 sin F12 y 5.4 N sin 146.3 F12 y 3.00 N F13 x F13 cos F13x 0.972 N cos 206.6 FNet F13 x 0.87 N F13 y F13 sin F13 y 0.972 N sin 206.6 FNet F13 y 0.44 N FNet YTot tan X Tot Y tan 1 Tot X Tot 1 2.56 N tan 5.36 N 25.5 X Tot F12 x F13 x X Tot 4.49 N 0.87 N X Tot 5.36 N YTot F12 y F13 y YTot 2.56 N X Tot 2 YTot 2 5.36 N 2.56 N 5.94 N 2 2.56 Quad II Adjust 2 25.5 180 154.5 FNet 5.94 N @154.5 Electric Field lines • Electric Field lines indicate the direction of the force due to the given field. • The lines point radially outward from a positive charge. • The lines point radially inward from a positive charge. Electric Field lines • Electric Field lines can bend if there is more than one charged particle. Use symmetry to help.