Metal Complexes: Ligands, Isomers, & Crystal Field Theory
advertisement

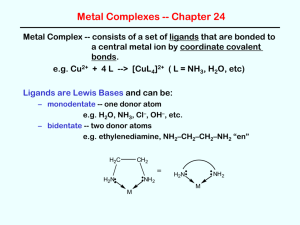
Metal Complexes -- Chapter 24 Metal Complex -- consists of a set of ligands that are bonded to a central metal ion by coordinate covalent bonds. e.g. Cu2+ + 4 L --> [CuL4]2+ ( L = NH3, H2O, etc) Ligands are Lewis Bases and can be: – monodentate -- one donor atom e.g. H2O, NH3, Cl–, OH–, etc. – bidentate -- two donor atoms e.g. ethylenediamine, NH2–CH2–CH2–NH2 “en” H2C CH 2 = H2N NH 2 NH 2 H2N M M Polydentate ligands – polydentate -- more than two donor atoms e.g. EDTA -- ethylenediaminetetraacetic acid (6 donor atoms) O O O C H2 C H2C N O C O CH 2 CH 2 C CH 2 O = EDTA4– N H2C C O O Chelate Effect Chelate effect -- complexes with bi- or polydentate ligands are more stable than those with similar monodentate ligands e.g. [Ni(en)3]3+ is more stable than [Ni(NH3)6]3+ 3+ NH 2 NH 2 H2N Ni H2N N H2 NH 2 Know Table 24.2! Writing Formulas of Complex Ions Metal ion first, then ligands. Charge outside brackets. total charge = sum of metal ion + ligands e.g. metal ion Cu2+ Co3+ Fe3+ ligand H2O NH3 CN– complex [Cu(H2O)]2+ [Co(NH3)6]3+ [Fe(CN)6]3– Nomenclature • 1. Name the ligands, put in alphabetical order. • 2. Add prefixes for ligands that appear more than once. • 3. Name the metal – Cationic complex; metal name – Anionic complex; metal prefix + ate • 4. Add oxidation state in roman numerals • 5. As separate word, write “ion” if not neutral, or list counterions (positive first, negative second). Examples: • Complex Name [Ni(CN)4]2– tetracyanonickelate(II) ion [CoCl6]3– hexachlorocobaltate(III) ion [CoCl2(NH3)4]+ tetraaminedichlorocobalt(III) ion Na3[Co(NO2)6] sodium hexanitrocobaltate(III) [CrCl2(en)2]2SO4 dichlorobis(ethylenediamine)chromium(III) sulfate Coordination Number and Structure Coordination # -- number of donor atoms attached to the metal center (a) Two-Coordinate Complexes -- linear structures Rare except for Ag+ e.g. [Ag(NH3)2]+ and [Ag(CN)2]– (b) Four-Coordinate Complexes -- two structural types Tetrahedral structures -- common for ions with filled d subshells, e.g. Zn2+ as in [Zn(OH)4]2– 2 OH Zn OH HO OH More Coordination Number and Structure (b) Four-Coordinate Complexes -- two structural types square planar structures -- common for d8 metal ions (Ni2+, Pd2+, Pt2+) and for Cu2+ e.g. 2 2+ NH3 H3N Cl Cl Cu Pt NH3 H3N Cl Cl (c) Six-Coordinate Complexes -- the most common! “always” octahedral structures, e.g. 3+ NH 2 Cl NH 2 H2N Ni H2N N H2 NH 3 NH 3 Cr NH 3 NH 2 Cl Cl Sample Problem 1. Give the name or formula, as required. Draw the structure of each complex. a) [AgI2]– b) [Co(en)3]3+ c) cis-dichlorobis(ethylenediamine)cobalt(III) chloride d) sodium tetracyanonickelate(II) Structural Isomers of Coordination Complexes • Linkage isomers pentaamminenitrocobalt(II) ion pentaamminenitritocobalt(II) ion • Coordination isomers pentaamminechlorocobalt(II) bromide pentaamminebromocobalt(II) chloride Isomerism; Overview 10 Stereoisomers of Coordination Complexes (a) Geometrical Isomers Cl Cl NH 3 Cr NH 3 NH 3 NH 3 Cl NH 3 Cr NH 3 NH 3 NH 3 cis Cl trans fac mer (b) Enantiomers Three common ways to get enantiomers: --chiral ligand --tetrahedral complex with 4 different groups --octahedral complexes with chelating ligands (and no mirror plane) Sample Questions Draw a clear, 3-dimensional structure of the geometrical isomer of Co(en)(NH3)2Cl2 that is optically active. (define any abbreviations) Excess silver nitrate is added to a solution containing 0.0522 mol of [Co(NH3)4Cl2]Cl. How many g of AgCl (FW = 143.3) will precipitate? Sample Questions Draw a clear, 3-dimensional structure of the geometrical isomer of Co(en)(NH3)2Cl2 that is optically active. (define any abbreviations) NH 2 NH 2 NH 2 Co Cl NH 2 NH 3 NH 3 Cl NH 3 Cr Cl NH 3 Cl Excess silver nitrate is added to a solution containing 0.0522 mol of [Co(NH3)4Cl2]Cl. How many g of AgCl (FW = 143.3) will precipitate? 7.48 g Crystal Field Theory (Bonding in Transition Metal Complexes) Metal complexes are usually highly colored and are often paramagnetic -- such facts can be explained by a “d-orbital splitting diagram” dz2 energy eg dx2-y2 D = crystal field splitting energy dxy dxz dyz t2g d orbitals of the metal ion in an octahedral field of ligands d orbitals of the free metal ion Shape and Directionality of the d Orbitals eg D t2g Crystal Field Strength The size of D depends on… • The nature of the ligand (ligand-metal bond strength!) “spectrochemical series” -- D decreases: CN– > NO2– > en > NH3 > H2O > OH– > F– > Cl– > Br– “strong field ligands” “weak field ligands” • The oxidation state of the metal (charge!) D is greater for M3+ than for M2+ • The row of the metal in the periodic table (size!) for a given ligand and oxidation state of the metal, D increases going down in a group e.g. D is greater in Ru(NH3)63+ than in Fe(NH3)63+ Colors of metal complexes are due to electronic transition between the t2g and eg energy levels d Orbital Splitting Diagrams for Octahedral Complexes dx2 dx2 dx2–y2 eg dx2–y2 eg large D “low spin” small D “high spin” dxy dxz dyz Fe(H2O)62+ t2g dxy dxz dyz Fe(CN)64– t2g High Spin vs. Low Spin CN– is a stronger field ligand than is H2O which leads to a greater D value (i.e. a greater d orbital splitting) As a result, • Fe(H2O)62+ is a “high spin” complex and is paramagnetic (4 unpaired electrons) while, • Fe(CN)64– is a “low spin” complex and is diamagnetic (no unpaired electrons) The CN– complex with the larger D value absorbs light of higher energy (i.e. higher frequency but shorter wavelength) OMIT … d orbital splitting diagrams for other geometries (i.e. tetrahedral and square planar) Sample Questions A complex [CoA6]3+ is red. The complex [CoB6]3+ is green. a) Which ligand, A or B, produces the larger crystal field splitting D? b) If the two ligands are ammonia and water, which is A and which is B? c) Draw the d orbital diagram of either complex, assuming it is “high spin.” How many unpaired electrons will it have? Give the order of increase of D in the following sets: a) Cr(NH3)63+, CrCl63–, Cr(CN)63– b) Co(H2O)62+, Co(H2O)63+, Rh(H2O)63+