SchuelkeSpr14
advertisement
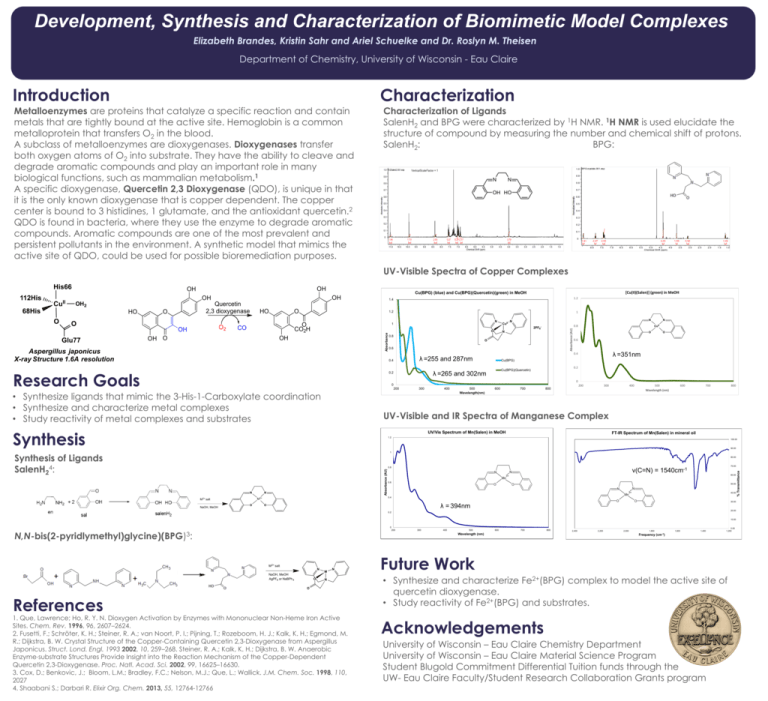
Development, Synthesis and Characterization of Biomimetic Model Complexes Elizabeth Brandes, Kristin Sahr and Ariel Schuelke and Dr. Roslyn M. Theisen Department of Chemistry, University of Wisconsin - Eau Claire Introduction Characterization Metalloenzymes are proteins that catalyze a specific reaction and contain metals that are tightly bound at the active site. Hemoglobin is a common metalloprotein that transfers O2 in the blood. A subclass of metalloenzymes are dioxygenases. Dioxygenases transfer both oxygen atoms of O2 into substrate. They have the ability to cleave and degrade aromatic compounds and play an important role in many biological functions, such as mammalian metabolism.1 A specific dioxygenase, Quercetin 2,3 Dioxygenase (QDO), is unique in that it is the only known dioxygenase that is copper dependent. The copper center is bound to 3 histidines, 1 glutamate, and the antioxidant quercetin.2 QDO is found in bacteria, where they use the enzyme to degrade aromatic compounds. Aromatic compounds are one of the most prevalent and persistent pollutants in the environment. A synthetic model that mimics the active site of QDO, could be used for possible bioremediation purposes. Characterization of Ligands SalenH2 and BPG were characterized by 1H NMR. 1H NMR is used elucidate the structure of compound by measuring the number and chemical shift of protons. SalenH2: BPG: Characterization of Metal Complexes UV-Visible Spectra of Copper Complexes OH OH OH O2 OH HO O O CO2H CO OH O 1.4 1.2 1.2 1 N 1 O OH N N N O 0.6 O 0.6 λ =351nm Cu(BPG) 0.2 Cu(BPG)(Quercetin) λ =265 and 302nm 0.2 O 0.4 λ =255 and 287nm N CuII 0.8 2PF6- 0.8 0.4 Research Goals CuII 0 0 200 • Synthesize ligands that mimic the 3-His-1-Carboxylate coordination • Synthesize and characterize metal complexes • Study reactivity of metal complexes and substrates 300 400 500 Wavelength(nm) 600 700 200 800 300 400 500 Wavelength (nm) 600 700 800 UV-Visible and IR Spectra of Manganese Complex Synthesis UV/Vis Spectrum of Mn(Salen) in MeOH FT-IR Spectrum of Mn(Salen) in mineral oil 1.2 100.00 90.00 1 Synthesis of Ligands SalenH24: 80.00 N N O 70.00 ν(C=N) = 1540cm-1 60.00 0.6 50.00 40.00 0.4 M 2+ M 2+ salt NaOH, MeOH Absorbance (AU) 0.8 30.00 λ = 394nm O 20.00 0.2 10.00 0 200 N,N-bis(2-pyridlymethyl)glycine)(BPG)3: 400 500 600 700 Wavelength (nm) M 2+ salt N NaOH, MeOH AgPF6 or NaBPh 4 O M N N O References 300 1. Que, Lawrence; Ho, R. Y. N. Dioxygen Activation by Enzymes with Mononuclear Non-Heme Iron Active Sites. Chem. Rev. 1996, 96, 2607–2624. 2. Fusetti, F.; Schröter, K. H.; Steiner, R. A.; van Noort, P. I.; Pijning, T.; Rozeboom, H. J.; Kalk, K. H.; Egmond, M. R.; Dijkstra, B. W. Crystal Structure of the Copper-Containing Quercetin 2,3-Dioxygenase from Aspergillus Japonicus. Struct. Lond. Engl. 1993 2002, 10, 259–268. Steiner, R. A.; Kalk, K. H.; Dijkstra, B. W. Anaerobic Enzyme⋅substrate Structures Provide Insight into the Reaction Mechanism of the Copper-Dependent Quercetin 2,3-Dioxygenase. Proc. Natl. Acad. Sci. 2002, 99, 16625–16630. 3. Cox, D.; Benkovic, J.; Bloom, L.M.; Bradley, F.C.; Nelson, M.J.; Que, L.; Wallick. J.M. Chem. Soc. 1998, 110, 2027 4. Shaabani S.; Darbari R. Elixir Org. Chem. 2013, 55, 12764-12766 800 2,400 2,200 2,000 1,800 1,600 1,400 0.00 1,200 Frequency (cm-1) Future Work • Synthesize and characterize Fe2+(BPG) complex to model the active site of quercetin dioxygenase. • Study reactivity of Fe2+(BPG) and substrates. Acknowledgements University of Wisconsin – Eau Claire Chemistry Department University of Wisconsin – Eau Claire Material Science Program Student Blugold Commitment Differential Tuition funds through the UW- Eau Claire Faculty/Student Research Collaboration Grants program % Transmittance O Absorbance HO Quercetin 2,3 dioxygenase [Cu(II)(Salen)] (green) in MeOH Cu(BPG) (blue) and Cu(BPG)(Quercetin)(green) in MeOH Absorbance (AU) OH