You suspect von Willebrand disease (vWD).
advertisement

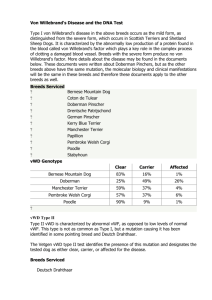
Automated vWF Assay vs. Ristocetin Cofactor Method in Diagnosis of von Willebrand Disease Group 3 Presentation Robert Ackerman Sean Cavanagh-Voss Jennifer Le Gonzalo Ortiz Shea Taylor Autumn Walker Evidence-Based Clinical Reasoning I USF Morsani College of Medicine February 2014 Case Description A 21 year old woman presents to your office for evaluation of abnormal menstrual periods. Her periods last from 7 to 9 days requiring up to 10 pads a day (excessive). On further questioning, she admits to easy bruising and nosebleeds. Family history reveals that her mother has had similar problems. You suspect von Willebrand disease (vWD). vWD is the most common inherited coagulation abnormality. Your laboratory informs you of a new automated assay for vW factor (vWF) activity. Since you have not heard about it, you decide to check what the published literature says about this new test and how does it compare to the established ristocetin cofactor method that you used to order to confirm the diagnosis of vWD. von Willebrand Disease (vWD) Deficiency in von Willebrand factor (vWF) – Two main functions of vWF: • Binds and stabilizes factor VIII • Mediates platelet adhesion to vascular injury site – Three classifications of vWD: • Type 1: partial, quantitative deficiency of vWF • Type 2: qualitative abnormalities of vWF (defective platelet adhesion or factor VIII binding) • Type 3: near absence of vWF Diagnosis of von Willebrand Disease Three main criteria: – Past medical history of excessive bleeding – Family history of excessive bleeding – Laboratory evaluation of vWF • Screening tests – Platelet count – Activated partial thromboplastin time – Bleeding or closure times • Confirmatory tests – von Willebrand factor antigen (VWF:Ag) – von Willebrand ristocetin co-factor test (VWF:RCo) – HemosIL von Willebrand Factor Activity Assay Confirmatory Lab Tests VWF:RCo – Ristocetin promotes binding of vWF and platelet glycoprotein Ib – Measures amount and rate of platelet aggregation – Challenges • Macroscopic slide method is semi-quantitative • Platelet aggregometer is labor intensive • High inter-assay and inter-laboratory variability Confirmatory Lab Tests HemosIL von Willebrand Factor Activity assay – Monoclonal antibody binds to platelet-binding site of vWF (glycoprotein Ib receptor) – Antibody is absorbed onto latex – Test measures turbidity that results from agglutination of latex The Question P Patients suspected of von Willebrand Disease I C O Automated assay for vWF activity Ristocetin co-factor test Detecting and classifying vWD Are automated assays done on patients suspected of von Willebrand Disease more or as accurate in the diagnosis of the disease compared with the ristocetin co-factor test? Search Strategy PubMed Clinical Queries, accessed through the USF Shimberg Library – Reliable, open database – Provides clinically relevant information that can help answer case questions Search “von Willebrand factor AND Ristocetin AND automated” Found a cross-sectional study – Abstract included details of interest and study objectives paralleled those of scenario Critical Appraisal Objectives Are the objectives of the study clearly stated? – Yes Design Is the study design suitable for the objectives? – Yes – The study was a prospective cross-sectional study, which is the best study design for assessing diagnostic tests. • Advantages: simple, ethically safe, relatively cheap • Disadvantages: establishes association, recall bias, confounders, unequal group sizes Design Who/what was studied? Design Was this the right sample to answer the objectives? – Yes – The authors selected a sample that avoids spectrum bias • All were suspected to have vWD; none were obviously diseased or obviously healthy – The patients were recruited consecutively indicating that selection bias is less likely. – Several blood types were represented. However, only two types of vWD were represented, indicating the full spectrum of vWD patients were not represented. Design Is the study large enough to achieve its objectives? Have sample size estimates been performed? – Uncertain • There are no indications that a power analysis was performed. • The sample size was relatively small (54 samples had complete data), but this is acceptable because vWD is relatively rare. • The authors admit that studies with more patients need to be done. Design Were all subjects accounted for? – Yes – They did not provide all the individual sample data but the tables all add up. Design Were all appropriate outcomes considered? – Yes – The authors considered all possible test result combinations Has ethical approval been obtained if appropriate? Design Was an independent blinded gold standard test applied to all subjects? – The gold standard test, ristocetin cofactor assay, was performed on all subjects. – It does not seem to have been independent • The authors performed both the gold standard and the test assay – It is unclear if the researchers were blinded, but considering the objectivity of the measures being compared, this may not be very critical. Measurement Is it clear what was measured, how it was measured, and what the outcomes were? – Agglutination of latex reagent at 405 nm – Decrease in light transmittance – Reference curves were utilized – MDA-180 & ACL TOP coagulation analyzers were utilized Measurement Are the measurements valid? Measurement Are the measurements reliable? – No conspicuous discrepancies exist amongst samples and equipment Measurement Are the measurements reproducible? – Not well described – They are likely to be reproduced due to quality control tests from the manufacturers, no significant differences between coagulation analyzers, and standard manufacturers’ protocol (and dilutions) applied to all assays Results Are the basic data adequately described? – Yes – Percentage agglutinations of each sample are clearly grouped and explained as to whether or not vWD is associated and, if so, which type Results Are the results presented clearly, objectively, and in sufficient detail to enable readers to make their own judgment? – Yes Results Are the results internally consistent, i.e. do the numbers add up properly? – Yes – Numerators and denominators included for most percentage calculations – Table 2 (previous slide) provided to show exactly how all cases were classified Analysis Are the data suitable for analysis? – Yes – Sensitivity and specificity desired for diagnostic study analyses Analysis Are the methods appropriate to the data? – Yes – For the type of diagnostic analysis needed, the agglutination methods are appropriate Analysis Are any statistics correctly performed and interpreted? – Hard to tell because no clear chart or individual patient data is provided – However, from the Table 2, we can calculate: • Specificity= 36/42= 86% • Sensitivity= 12/12= 100% – Positive predictive and negative predictive values provided because the true spectrum of patient population is not represented Discussion Are the results discussed in relation to existing knowledge and on the subject and study objectives? – Yes – The results explicitly mention the current gold standard of testing and the relation of the new method. – They briefly mention the relation of vWD and blood types, Factor VIII activity and PTT as well. – Furthermore, the results are provided as evidence to support the study objective, which was to analyze a new, quicker, easier test to screen for (and ultimately diagnose) vWD that could mitigate the reliance upon more complex tests, such as the Ristocetin Cofactor tests, to potentially save time and money. Discussion Is the discussion biased? – The discussion does not appear to be biased, but it is unknown if the researchers have a conflict of interest – They clearly favored the implementation of the new test, however, the data supported their claims of quality. Interpretation Are the authors’ conclusions justified by the data? – Yes – The data had 100% sensitivity and over 85% specificity, which supports the claim that the new test is a viable screening alternative. – However, the study was small, there was still sufficient heterogeneity, and all types of results were found, including normal. Interpretation What level of evidence has this paper presented (using CEBM levels)? – Level 1b or 1c • Study was a “validating” (not exploratory) study • Had good reference standard application • Absolute SnNout (sensitivity=100%) – Grade A recommendation • consistent with a level 1 study OCEBM Levels of Evidence Working Group. "The Oxford 2009 Levels of Evidence". Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/?o=1025 Interpretation Does this paper help me answer my problem? – Yes, it does, at least preliminarily. – I can utilize this new test with my patient (as long as it is cost-efficient) to rule out vWD. However, if the results are abnormal, I will have to retest utilizing the gold standard of the Ristocetin Cofactor test to be certain. How do you rate this paper? 8 – It seems to provide useful information, but with a limited patient population and data set, I cannot be certain of the validity of the results Case Review A 21 year old woman presents to your office for evaluation of abnormal menstrual periods. Her periods last from 7 to 9 days requiring up to 10 pads a day (excessive). On further questioning, she admits to easy bruising and nosebleeds. Family history reveals that her mother has had similar problems. You suspect von Willebrand disease (vWD). vWD is the most common inherited coagulation abnormality. Your laboratory informs you of a new automated assay for vW factor (vWF) activity. Since you have not heard about it, you decide to check what the published literature says about this new test and how does it compare to the established ristocetin cofactor method that you used to order to confirm the diagnosis of vWD. Diagnosis of von Willebrand Disease Three main criteria: – Past medical history of excessive bleeding • Periods last from 7 to 9 days, requiring up to 10 pads per day • Easy bruising • Nosebleeds – Family history of excessive bleeding • Mother has had similar problems – Laboratory evaluation of vWF Diagnosis of von Willebrand Disease Laboratory evaluation of vWF – Follow Figure 2 to determine procedure for ordering tests: Implementation Can any necessary change be implanted in practice? – Yes – As long as the new test is readily available and cost-effective, it would likely be a favored alternative Implementation What aids to implementation exist? – Technology, marketing, and coding. Since technology is so advanced, it should be relatively easy to order the required supplies and have the tests available “inhouse” in a short period of time. – With marketing, more people could be informed about the new, “easy” test and opt to have it done, especially if it is faster, cheaper, and easier than the old method. – Depending on the medical coding being used in each clinic or hospital, a new code could be created to bill the patient or the patient’s insurance for the test. Implementation What barriers to implementation exist? – Insurance companies might be reluctant to pay for new technology, especially if it implicates further disease states that it will be responsible for covering. – Some physicians and/or facilities may not want to try something new, especially if they are used to the “old ways” of doing things. – Depending on how much time and money is really saved by the new method, insurance companies may not want to cover it, especially if the patients end up having a second test done as well. Implementation Are my patients the same as the patients tested? – Yes, approximately. – The mean age was 37 (range 2-89 years), and my patient is 21, so she falls within the range of patients tested. Implementation Will the test improve diagnosis in my patients? – It may not improve the diagnosis per se, however, it may make the process easier and completed more quickly and easily, which could potentially correlate to savings of money and time. – Therefore, if I had several patients who needed to be tested, it might improve their diagnosis in that they get it more quickly and thus receive proper treatment more quickly as well. References 1. Salem, RO, Van Cott, EM. A new automated screening assay for the diagnosis of von Willebrand disease. Am J Clin Pathol 2007; 127:730-735. 2. De Vleeschauwer, A, Devreese, K. Comparison of a new automated von Willebrand factor activity assay with an aggregation von Willebrand ristocetin cofactor activity assay for the diagnosis of von Willebrand disease. Blood Coagul Fibrinolysis 2006; 17:353-358.