Lewis Structures
advertisement

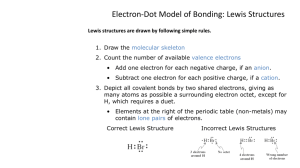
Ch. 6 Lewis Structures and Molecular Geometry How to draw Lewis Structures, or dot diagrams of e- bonding Only the Valence e- are involved Most atoms strive to get 8 e- in their outer principal electron layer. This is the “Octet Rule.” Here’s how many valence e- each group has. Write them in on your chart! In a Lewis Structure Each dot represents 1 electron. Each line represents 2 shared electrons in a covalent bond. Sometimes the lines are shown as dots; see this diagram: How to draw Lewis Structure for the OCl- (bleach) ion. 1. Count the valence e- available. Add electrons for negative charges. Subtract electrons for positive charges. Step 2: Draw a “skeleton” structure using single bonds (lines) for pairs of shared electrons. Step 3 Distribute the rest of the e- around the atoms so each atom has 8 electrons. There are 3 things that can occur with your electrons on the central atom: 1. There are just enough e- to go around. Every atom has 8 and H atoms have 2. 2. If there aren’t enough e- to go around, then move a pair of e- next to an existing single bond to make a double or triple bond. 3. If there are too many e-, then the atom may have an “Expanded Octet.” Put the extra epairs on the central atom. An example of an “expanded octet” Here’s the Lewis Structure for XeF4 Important Tips: Carbon is usually a central atom, while H, O, and the Halogens are usually terminal atoms with ONLY one single bond. The FIRST atom listed in the chemical formula is usually the central atom. Carbon always has 4 lines (4 covalent bonds) coming off of it. Draw the Lewis Structure for CH3OH, methanol. Draw the Lewis Structure for the sulfite ion, SO32- Draw the Lewis Structure for the Nitrate ion, NO3 . Stuck? Look at the “Important Tips” we just covered! Exceptions to the Octet Rule: Some light elements don’t have 8 e- on the central atom. Examples: H, Li, Be, and B Lewis Structures for BeF2 and BF3 Part 2: Predicting Molecular Geometry (Shapes) Molecular shapes can be predicted using the Valence Shell Electron Pair Repulsion, VSEPR principle. All e- pairs try to get as far away from each other as possible. You must draw the Lewis structure before you can predict the geometry. A good reference chart: How to predict the “Repulsion Angles” in molecules: Add up the number of “forces” pushing away from the central atom. Each lone e- pair and atom(s) bonded to the central atom, count as a “force.” Double or Triple bonds behave like just one “force.” The 3 basic “Repulsion Angles” 2 Forces: make a Linear molecule with 180 ° angles off the central atom. 3 Forces: repel in a Trigonal Planar pattern with 120 ° angles. 4 Forces: repel in a Tetrahedral pattern with 109.5 ° angles. The names of the possible shapes. I presented only 3 possible repulsion angles, but 5 possible molecular shapes exist given these 3 angles. The molecule’s shape is named after the 3D arrangement of the atoms. Unshared e- pairs (dots) on the central atom influence the shape but are not seen. How do you tell if a molecule is “polar?” A molecule is “polar” when it has more electrons on one side than on the other. This means it has a negative side with more electrons and a positive side with less electrons. Generally, if a molecule is perfectly symmetrical with no unshared e- pairs, then it is non-polar. Unshared pairs usually make it polar. If the terminal atoms are different, then it's polar (different charge on each end) What is the molecular geometry of BF3 ? 120 ° angles, Trigonal Planar, non polar What is the geometry of a water molecule? 105 ° angles, Bent, very polar. Polar or Nonpolar?