Chapter 20: Electrochemsitry
advertisement

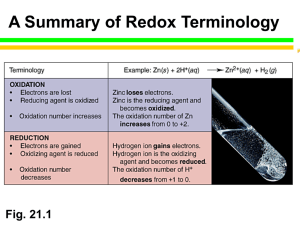
Chapter 20: Electrochemsitry A.P. Chemsitry 20.1 Oxidation-Reduction Reactions • Oxidation-reduction reactions (or redox reactions) involve the transfer of electrons from one species to another. In other words, the oxidation state of one or more substances in the reaction changes. The transfer of electrons inherent in an oxidation-reduction process can be used to produce energy in the form of electricity. Electrochemistry is the study of the relationships between chemical reactions and electrical energy. 20.2 Balancing Oxidation-Reduction Equations • A half-reaction is an equation that shows either oxidation or reduction alone. Reduction half-reaction Zn (s) Zn2+(aq) + 2 e- Oxidation half-reaction Balancing steps 1. 2. Divide the equation into two incomplete half-reactions, one for oxidation and the other for reduction. Balance each half-reaction. [Assuming an acidic solution] 1. 2. 3. 4. 3. 4. 5. 6. 7. First, balance the elements other than H and O Next, balance the O atoms by adding H2O [OH- for basic solution] Then, balance the H atoms by adding H+. [H2O for basic solution] Finally, balance the charge by adding e- to the side with the greater overall positive charge. Multiply each half-reaction by an integer so that the number of electrons lost in one half-reaction equals the number gained in the other. Add the two half-reactions, and simplify where possible by canceling species that appear on both sides of the equation. Check the equation to make sure that there are the same number of atoms of each kind on both sides and the same total charge on both sides. Neutralize any H+ ions in the equation with OH- ions, adding the same number of OH- ions to the opposite side of the equation. (The combination of an H+ and an OH- produces a water molecule.) Cancel any resulting water molecules with water molecules on the opposite side of the equation. Reduction Half-Reaction Equation Oxidation Half-Reaction Equation Add electrons to each side to balance charge Multiply to get same electrons in each half reaction Then combine the half-reactions Check to make sure there are the same number of each type of atom on each side of the equation 20.3 Voltaic Cells Salt Bridge • In a voltaic cell the two half-reactions are made to occur in separate compartments (half-cells) • The electrode at which oxidation occurs is called the anode; in this case, the zinc electrode. The electrode at which reduction takes place is called the cathode; in this case, the copper electrode 20.4 Cell EMF Under Standard Conditions • The potential difference that drives electrons through the wire in a voltaic cell is called the electromotive force or emf. For a voltaic cell the emf is denoted Ecell and referred to as the cell potential. • The value of a cell potential depends on what half-reactions are taking place in the two compartments of the cell. The cell potential measured under standard conditions, E°cell (25°C, 1 M concentrations, and 1 atm pressures), is the standard cell potential or standard emf. Calculating EMF The standard reduction potentials for the various half-reactions are measured against a standard hydrogen electrode (SHE). The halfreaction of interest and the SHE, both under standard conditions, are made into a voltaic cell, as shown above, and the cell potential is measured experimentally. The standard potential of the standard hydrogen electrode's half-reaction is arbitrarily assigned a value of zero, so the measured potential corresponds to the half-reaction being evaluated. Sample Calculation What E0 means • The standard reduction potentials in Table 20.1 can be used to compare the oxidizing power or reducing power of a substance. The more positive the value of E°red for a species, the more readily it undergoes reduction and the better oxidizing agent it is. As E°red becomes more negative, the species on the right side of the arrow becomes a stronger reducing agent. 20.5 Spontaneity of Redox Reactions • Our ability to predict the spontaneity of a chemical reaction by calculating a standard cell potential points to a relationship between the sign of E°cell and the sign of G° , the standard change in Gibbs free energy. Sample Calculation 20.6 Effect of Concentration on EMF • Just as the values of G and G° were related, the values of E and E° are related. The Nernst equation derives from the relationship between G and G° . • Substituting - nFE for G gives • Solving for E gives • The Nernst equation is most commonly expressed in terms of log (base 10) rather than ln (natural log). • When the temperature is 298 K, this expression simplifies to Concentration Calculation Calculate E0 20.7 Batteries • A battery is a self-contained source of electrochemical energy made from one or more voltaic cells. Ordinary flashlight batteries consist of a single voltaic cell, while car batteries are six identical voltaic cells connected in series. It is worth noting that electrochemistry is one of only a very few commercially viable methods of generating electricity. 20.8 Corrosion • Corrosion is the undesirable oxidation of a metal. A familiar example of corrosion is the rusting of iron. Iron metal is oxidized to Fe2+ by oxygen. • The Fe2+ is then further oxidized to Fe3+ in a hydrated form of Fe2O3, what we know as rust. • To undergo these oxidationreduction reactions, iron must be in contact with oxygen and water. Electrolysis • • A voltaic cell is one in which a spontaneous chemical reaction is used to generate a voltage. Electrolysis is the use of a voltage to drive a nonspontaneous reaction. Reactions that are driven by an externally supplied voltage are called electrolysis reactions, and electrochemical cells designed for the purpose of carrying out electrolysis reactions are called electrolytic cells Sodium metal is produced commercially by electrolysis of molten sodium chloride. Electrodes are immersed in molten sodium chloride, and a voltage source drives electrons from the anode to the cathode. Sodium is reduced at the cathode to molten sodium metal. Chloride ions are oxidized to chlorine gas at the anode.