10. Density of Fluids
advertisement

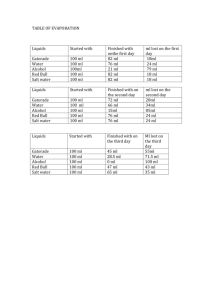
Density 10 Find the Density of Fluids: The Cocktail Lab ©Sue Boudreau 12.7.2011 Investigate the density of fluids with direct measurement and floating and sinking. Apply your learning to cocktails and a cool desktop game! 1. Define a “FLUID”. Give some examples. 2. Look at the cool color drop desk toy. As you do the lab, think about how it works and what the liquids are. 3. What is the density of water from our previous lab (give units)? ____________ 4. How much will 10ml of water weigh? ___________ 5. What volume of water do you have if it weighs 40g? _______________ 6. You have 100ml of 3 different liquids and an empty container. Work out the density of those liquids using the electronic scales and the tare containers. Data table: 7. Add 7g of salt to 70ml of warm water. Swirl it in a conical flask until it all dissolves. 8. Re-measure the volume of your salt water. Write it here: _________________ 9. What is the weight of the salt water: _____________ 10. What is the density of tsalt water? Show your work: 11. Layered cocktails have the densest layer at the bottom. The less dense liquids float on top. What order would you make a cocktail of the liquids we used today, including salt and fresh water? Put “1” by the first, “2” by the next, etc. 12. Draw & label the cocktail that worked: 13. How does the color drop desktop toy work? Use the words ‘density’ and ‘miscible’, ‘immiscible’ fluids in your answer. EXTRA CREDIT: Make a delicious, non-alcoholic cocktail. Photograph & label it. Or make a desktop color drop toy from home made materials. Bring it in. Teacher Notes: A beautiful, intriguing lab that really engaged students and builds on prior knowledge of measuring density in solids. Demos: Desktop ‘color drop’ fluid toys – about $4.00 each online or ask kids to bring in. For how salt dissolves in water without changing the volume much: Large plastic graduated cylinder Metal balls Beads For the big class cocktail: 250ml glass graduated cylinder or a tall glass, one per class or wash between. Dyed corn syrup Dyed rubbing alcohol, Saturated salt solution Dyed fresh water Cooking oil Pipette to dribble freshwater on top of salt water (very miscible, has to be done very gently.) Equipment per table: Tub put equipment and chemicals in. 2 electronic scales 100ml of dyed corn syrup in a closed bottle. (Mark the level with sharpies so they can be refilled next year.) 100ml of dyed 91% alcohol in a closed bottle 100ml of cooking oil in a closed bottle Empty closed bottle for tare. 1 cup with salt in it. 1 empty container for measuring salt into. 1 teaspoon. 100ml graduated cylinder (plastic is much better as it doesn’t break) 250ml conical flask Plastic water container or beaker. Management Tips: Before every wet lab, have everything except for lab sheet and clipboards off desks. Backpacks and trip hazards away. At start of class, have tubs with equipment on the counters or at front if fiddling is an issue. Safety, Hygiene, Cleanliness Tips: Trip hazards, oil and water slip hazards. Not drinking the alcohol – Rubbing alcohol is poisonous. Allow 10 minutes at end to go over answers and to demo the cocktail and discuss the desktop games. 10 minutes before the end of class, clean-up. Have tubs with clean, dry equipment and chemicals at the end of desks.