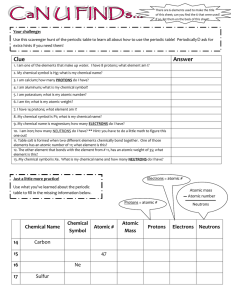
Element Name
advertisement
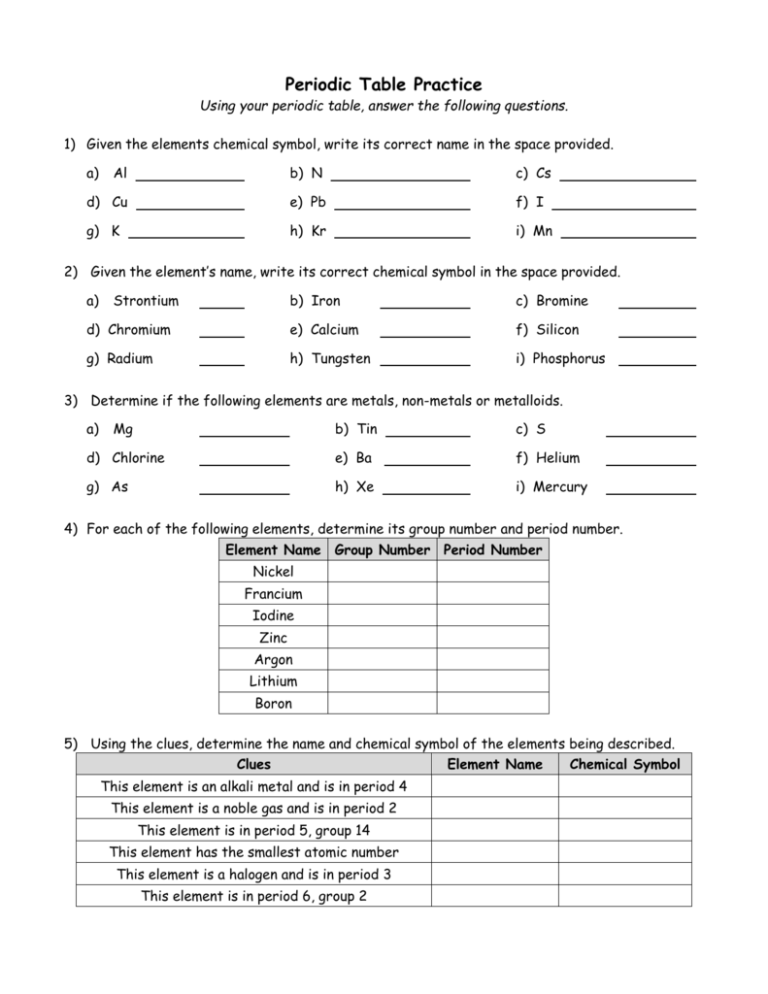
Periodic Table Practice Using your periodic table, answer the following questions. 1) Given the elements chemical symbol, write its correct name in the space provided. a) Al b) N c) Cs d) Cu e) Pb f) I g) K h) Kr i) Mn 2) Given the element’s name, write its correct chemical symbol in the space provided. a) Strontium b) Iron c) Bromine d) Chromium e) Calcium f) Silicon g) Radium h) Tungsten i) Phosphorus 3) Determine if the following elements are metals, non-metals or metalloids. a) Mg b) Tin c) S d) Chlorine e) Ba f) Helium g) As h) Xe i) Mercury 4) For each of the following elements, determine its group number and period number. Element Name Group Number Period Number Nickel Francium Iodine Zinc Argon Lithium Boron 5) Using the clues, determine the name and chemical symbol of the elements being described. Clues This element is an alkali metal and is in period 4 This element is a noble gas and is in period 2 This element is in period 5, group 14 This element has the smallest atomic number This element is a halogen and is in period 3 This element is in period 6, group 2 Element Name Chemical Symbol Use the table below to practice with the basics of your periodic table. Complete all blank spaces with the information required. Assume the atom is neutral unless indicated otherwise. Element Name Oxygen Element Symbol Atomic Number Atomic Mass Number of Protons Number of Electrons Number of Neutrons 5 9 Sc 23 33 0 Co Manganese Re 71 54 Te Ag 86 Pb 103 Thallium 62 Os 14 28 Ne Things to remember: Atomic number = # of protons In a neutral atom # protons = # electrons # protons + # neutrons = Mass # Mass # - # protons = # neutrons