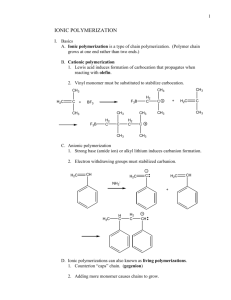
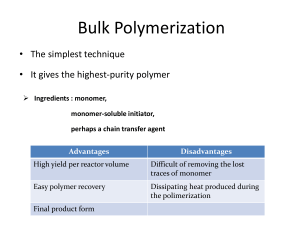
Addition Polymerization
advertisement
- Organic Reactions Mr. Shields Regents Chemistry U17 L03 Organic Reaction Types There are in fact so many types of organic rxn’s It would be impossible to review them all. Therefore we’re going to focus on just 7: - Substitution - Addition - Fermentation - Elimination - Esterification - Saponification - Polymerization (Condensation & Addition) Substitution Rxns • Any reaction in which one atom is replaced by another • Used to place a halogen onto an alkane • The products always are a halocarbon and the acid of the halogen (ex: hydrobromic acid) • Need ultraviolet light to initiate the reaction – Provides the energy of activation needed to form the excited state H H H C H H C H H H hv + Cl2 C H Cl C H H + HCl Substitution Rxns What are the products formed in the following rxn? CH3CH3 + Br2 CH3 CH2Br + HBr sunlight (What is the purpose of the sunlight?) Let’s look at how this reaction occurs? 1. Br-Br 2Br uv 2. R-H + Br H-Br + R A free radical 3. R + Br2 R-Br + Br Substitution Rxns Another example of a substitution reaction is The replacement of a halogen with a hydroxyl group CH3CH2CL + NaOH(aq) CH3 CH2OH + NaCl Or the replacement of a halogen with an amine group CH3CH2CL + NH3 CH3 CH2NH2 + HCl Elimination Rxns • Any reaction in which atoms are eliminated from another molecule • This can be done by – Elimination of H2 – Elimination of HX Elimination Rxns -Loss of H2 - This process is often referred to as Dehydrogenation HH H-C-C-H HH H2C=CH2 + H2 Heat, catalyst Elimination Rxns -Loss of HX (X = a halogen) -Also known as dehydrohalogenation HH H-C-C-H (g) + Heat HX H2C=CH2 + HX (g) Addition • Takes place with unsaturated compounds which are usually more reactive than saturated compounds – Can take place with both Double and Triple bonds – Two atoms are added across the electron rich double bond • What are some examples of molecules that can be added? – – – – X2 H2 HX H 2O Addition • Addition of halogen – Normally occurs dissolved in a solvent such as CCL4 – Alkenes form dihaloalkanes – Alkynes produce dihaloalkenes or tetrahaloalkanes H H H H C H C H + Cl2 C H C Cl Cl H 1,2-dichloroethane Addition • Addition of Hydrogen – Catalysts normally used such at Pt, Pd or Ni • Known as Hydrogenation – Alkene becomes an alkane H2C=CH2 + H2 Heat, catalyst HH H-C-C-H HH Addition • Addition of Hydrogen Halides (HX) – HX = HCl, HBr, HI (Not HF!) – Alkene becomes an alkyl Halide – Alkynes form Monohalo alkenes or dihaloalkanes with the halogens on the same carbon H2C=CH2 + HX HH H-C-C-H HX HX HC=CH + HX H-C-C-H + HX H-C-C-H HX HX Addition • Addition of Water - Water adds across a double bond to form an alcohol - Water can add across a triple bond to form a diol H2C=CH2 + H-OH HH H-C-C-H H OH HH HC=CH + H-OH H-C-C-H + HOH H-C-C-H H OH HO OH Esterification • Alcohol + Organic Acid = Water + Ester • Used to make perfumes, scents and flavors • Combination rxn which involves dehydration (Loss of water). • The alcohol becomes the alkyl group & the acid becomes -oate alcohol propyl ethanoate acid From the alcohol From the acid Aspirin – Made by Esterification HO C=O OH Salicylic Acid (An alcohol and acid) + H-O-C-CH3 O Acetic acid HO C=O O-C-CH3 O Acetyl Salicylic Acid (Common Name) “Aspirin” Name the Esterification Products CH3CH2OH + HCOOH CH3CH2COOH + CH3CH2CH2OH Fermentation • Fermentation is the process by which glucose is broken down by an enzyme (a catalyst) in the absence of oxygen into an alcohol and carbon dioxide • One enzyme used is Zymase (Found in baker yeast) – If Zymase is used the alcohol produced is ethanol • The oldest chemical reaction practiced by man – Dates back to at least 6000 B.C. – In place of glucose, starches from grains can be used. Hence the name grain alcohol C6H12O6 Zymase Glucose 2C2H5OH + 2CO2 Ethanol Carbon dioxide Saponification • Another very old chemical reaction practiced by man • The hydrolysis of the ester bonds (back to acid + alcohol) in triglycerides using an aqueous sol’n of a strong base to form carboxylate salts and glycerol • Triglycerides,from fats, and a strong base (KOH or NaOH) – Products are soap and glycerol (a triol) Carboxylate salt An ester O CH2-0-C-(CH2)14CH3 | O CH2-O-C-(CH2)14CH3 | O CH2-0-C-(CH2)14CH3 A TRIGYCERIDE + 1,2,3-propanetriol O CH2-0H K+ -O-C-(CH2)14CH3 | O 3KOH CH -OH K+ -O-C-(CH ) CH 2 2 14 3 + | O CH2-0H K+ -O-C-(CH2)14CH3 GLYCEROL 3 SOAP MOLECULES Polymers The joining together of many smaller repeating Units to form a very high MW molecule - Polymers range from 10,000 amu to more than 1,000,000 amu The small repeating units used to build the polymer are known as monomers Monomers Sometimes just one monomer is used to make the Polymer (example: ethylene (a) to form polyethylene) a a a a a a a And sometimes two monomers alternate are used to form an alternating polymer (ex: Nylon or Polyesters) a b a b a b a Natural polymers Example of “natural” polymers in nature abound: Some examples are: Wool Cotton Starch Protein Cellulose Polymerization There are two methods we’ll look at for the Production of Polymers: - Addition polymerization - Condensation polymerization Addition Polymerization -All the atoms present in the monomer are retained in the polymer in Addition Polymerization -This type of reaction involves monomers with double or triple bonds -An initiator is required to produce a free radical -A very reactive substance having a free e-Peroxides are typically used to produce this free radical Peroxide Rad Free radical induced addition polymerization of Ethylene to form polyethylene Rad Rad Free radical induced addition polymerization of Styrene to form polystyrene Monomer Notice loss of electron pair to form Connecting bonds in polymer Addition Polymer Condensation Polymerization - Monomers that join together by the loss of water - each monomer has two functional groups that are the same - monomer 1 and monomer have functional groups that are different - reaction occurs between the two pairs of dissimilar functional groups Let’s look at some examples … One example of Condensation Polymerization - Dacron Di-Acid Monomer A Di-Alcohol Monomer B Formed by loss of water A polyester Condensation Polymerization Nylon Formed by loss of water An amide group A Polyamide I’m Done! You’re Done! WE Made it to the End! Only the regents is left !!