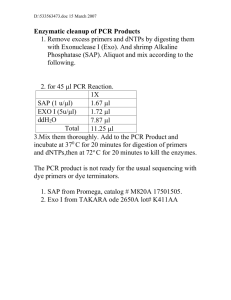
Bild 1
advertisement

Lab safety Documentation, GLP Practical tips; primers and PCR Lab safety - wash your hands - EtBr - sterile technique Lab safety - Use Labcoats - No food in the lab - Common sense! Lab safety Documentation, GLP Practical tips; primers and PCR What can an experiment looks like? Title should be descriptive! Background information why do you do this experiment? Methods all steps you do in the lab, include all calculations made in preparing solutions Results and conclusion Overview Day 1. Mutation PCR reaction Day 2. DpnI digestion Day 5. Do small-scale expression test Analysis: SDS-PAGE Analytical agarose gel Day 6. Analysis: SDS-PAGE Transformation to TOP10 Day 7. Large scale fermentation Day 3. Day 8. Purification (I) Day 9. Purification (II) Pick 3 colonies, patch and inoculate o/n cultures Day 4. Plasmid miniprep Analytical PCR and inoculate o/n cultures Analysis: SDS-PAGE, Protein concentration Day 10. Activity test. Where to store stuff at room temperature: DNA loading dye 10 mM Tris pH 8.5 5xSB at 4C: agar plates with bacteria on them (if you want to keep them) cultures that you want to use as inoculum -20C: dNTP (keep on ice whenever out of freezer) 10xPfu buffer 10xTaq buffer template primers DNA ladder Amp plasmids samples taken out for SDS-PAGE Lab safety Documentation, GLP Practical tips; primers and PCR Primers Materials: tube with dried primer. On the tube label, you can see how many nmoles there are inside. Experiment: Stock solution (100 µM): Spin the tube a few seconds. Open carefully and add 10 x (nmoles of primer) µl of autoclaved distilled water Rehydrate for 2 min (ie. leave on bench). Tap the tube. Working solution (20 µM): 20 µl 100 µM stock solution 80 µl autoclaved distilled water Pipetting enzymes in 50% glycerol tip just below surface look at the tip!!!