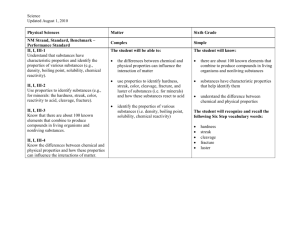
Mineral Groups - geo1stsem2012

Mineral Groups
Mineral Groups
Element Abundances
SILICATES
All others: 1.5%
Common cations that bond with silica anions
Silica
(SiO
4
) 4-
Mineral Groups
Non-ferromagnesian
Silicates (K, Na, Ca, Al)
Ferromagnesian
Silicates (Fe, Mg)
Oxides
Carbonates
Sulfides/sulfates
Native elements
Silicates
Actinolite (Tremolite) Ca
Subclass: Amphibole
2
(Mg,Fe)
5
Si
Streak: White
8
O
22
(OH)
2
Lustre: Vitreous Hardness: 5 – 6
Density: 3.0 - 3.4
Habit: Aggregate of acicular grains
Colours: Greenish - white
Crystal System: Monoclinic
Cleavage: Uneven
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Habit and colour are best indicators.
Associations:
Metamorphosed calcareous sediments
Comments:
Higher in Fe than tremolite.
Silicates
Antigorite (Serpentine) Mg
3
Subclass: Phyllosilicate
Hardness: 2.5 - 3.5
Density: 2.6
Habit: Lath-shaped crystals.
Colours: Shades of green
Si
2
O
5
(OH)
4
Streak: White
Lustre: Greasy or waxy
Crystal System: Monoclinic
Cleavage: Perfect basal
Fracture: Splintery or conchoidal
Magnetism: None
Reaction with HCl: Unknown
Identification in hand sample:
Lustre, colour and habit are indicative.
Associations:
Formed by hydrothermal action of mafics and ultramafics. Associations may include talc, calcite, brucite, chlorite, magnetite and chromite.
Silicates
Augite (Ca,Na)(Mg,Fe,Al,Ti)(SiAl)
2
O
6
Subclass: Pyroxene
Hardness: 5.5 – 6
Density: 3.2 - 3.5
Habit: Small blocky grains.
Colours: Dark-greeny black colour
Streak: White or gray
Lustre: Vitreous
Crystal System: Monoclinic
Cleavage: Good / Uneven
Fracture: Uneven to conchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Colour, cleavage and hardness are indicative of a pyroxene. Augite has 2 cleavage planes at 90°.
Associations:
Found in mafic to ultramafic rocks, and in high-grade metamorphs.
Industrial / ecomonic uses:
None.
Silicates
Biotite K(Mg,Fe)
3
Subclass: Phyllosilicate
(AlSi
3
O
10
)(OH)
2
Hardness: 2 – 3
Density: 2.7 - 3.4
Habit: Micaceous
Colours: Brown, black, reddish brown
Streak: Colourless
Lustre: Vitreous
Crystal System: Monoclinic
Cleavage: Perfect basal
Fracture: Uneven
Magnetism: None
Reaction with HCl: Very weak
Identification in hand sample:
Colour and habit
Associations:
A very common mineral found in igneous, metamorphic and sedimentary rocks.
Industrial / ecomonic uses:
Altered into vermiculite, biotite is used as an insulation material and as filler in certain building supplies.
Silicates
Chalcedony (Micro/Cryptocrystalline Quartz) -- SiO
Subclass: Framework
Hardness: 7
Density: 2.7
Streak: White
Lustre: Vitreous / almost waxy
Habit: Aggregate of very tiny SiO
2
Colours: White/gray or any colour grains.
2
Crystal System: Hexagonal
Cleavage: None
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Identified by hardness and texture/habit.
Associations:
Abundant in felsic rocks.
Industrial / ecomonic uses:
Used for making glass and as a source of Silicon.
Silicates
Chlorite (Mg,Fe,Al)
3
(Si,Al)
4
O
10
(OH)
2
Subclass: Phyllosilicate
Hardness: 2 - 2.5
Density: 2.6 - 3.3
Streak: Greenish white to white
Lustre: Vitreous to somewhat pearly, waxy, dull
Colours: Greenish-black (typical)
Crystal System: Monoclinic
Cleavage: Perfect {001}
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Colour and its low hardness are distinguishing characteristics.
Industrial / ecomonic uses:
None, however chlorite schist sometimes does have ornamental uses.
Silicates
Chrysotile (Serpentine) Mg
3
Subclass: Phyllosilicate
Si
2
O
5
(OH)
4
Hardness: 2.5
Streak: White
Lustre: Silky Density: 2.5
Habit: Fibrous
Colours: Shades of green
Crystal System: Monoclinic
Cleavage: None
Fracture: Uneven
Magnetism: None
Reaction with HCl: Unknown
Identification in hand sample:
Habit and colour and most indicative properties.
Associations:
Found by hydrothermal alteration of mafics and ultramafics. Associated with talc, calcite, brucite, chlorite, magnetite and chromite.
Industrial / ecomonic uses:
Used in 98% of world's production of asbestos.
Silicates
Epidote Ca
Subclass: Sorosilicate
2
(Fe,Al)
3
(SiO
4
)
3
(OH)
Hardness: 6 – 7
Density: 3.4 - 3.5
Streak: White
Lustre: Vitreous
Habit: Aggregate of small grains, striated faces, small but visible crystals
Colours: Greeny, yellow-green, black
Crystal System: Monoclinic
Cleavage: Perfect
Fracture: Uneven
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Slight colour difference between this mineral and olivine, but only under magnification (where details in the crystals can be seen). Also indicitive is the presence of a cleavage plane.
Associations:
Common accessory mineral in many regional and contact metamorphed rocks, particularly in more iron-rich rocks.
Industrial / ecomonic uses:
Semiprecious gemstone
Silicates
Garnet (Pyrope, Grossular,
Almandine)(Mg,Fe,Ca)
3
Hardness: 6.5 – 7
Density: 3.8
Habit: Small, well-formed crystals
Colours: Darker reddy colours
Al
2
(SiO
4
)
3
Streak: White
Lustre: Vitreous
Crystal System: Isometric
Cleavage: None
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Crystal habit, colour and hardness.
Industrial / ecomonic uses:
Abrasives (particularly sandpaper), semi-precious gemstone.
Silicates
HornblendeCa
2
(Mg,Fe)
4
Al(Si
7
Al)O
22
(OH,F)
2
Subclass: Amphibole
Hardness: 5 – 6 Streak: White or gray
Density: 3.3 - 3.4
Lustre: Vitreous
Habit: Intergrown crystals, prismatic character of grains.
Colours: Black
Crystal System: Monoclinic
Cleavage: 2 at 56° and 124°
Fracture: Uneven / conchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Prominent striations, prismatic crystals and angle on cleavages is distinguishing.
Associations:
Forms in igneous rocks, and in the metamorphic rock amphibolite.
Industrial / ecomonic uses: Ornamental.
Silicates
Muscovite KAl
2
(AlSi
3
O
10
)(OH)
2
Subclass: Phyllosilicate
Hardness: 2.5 – 4
Density: 2.8 - 2.9
Habit: Micaceous
Colours: Colourless, to white or gray
Streak: Colourless
Lustre: Vitreous
Crystal System: Monoclinic
Cleavage: Perfect basal
Fracture: Uneven
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Colour and habit are best indicators.
Industrial / ecomonic uses:
Used in the electronics industry in the manufacture of capacitors, transistors, insulators and certain window types. Also used as a filler in building materials.
Silicates
Olivine (Forsterite (Mg) and Fayalite (Fe))Mg
2
Subclass: Nesosilicate
SiO
4
Hardness: 6.5 – 7 Streak: Colourless
Density: 3.3 - 4.3
Habit: Granular
Lustre: Vitreous
Colours: Pistachio green, greenish yellow brown
Crystal System: Orthorhombic
Cleavage: Imperfect
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: Very weak
- Fe
2
SiO
4
Identification in hand sample:
Pistachio-green colour and granular habit.
Associations:
Mafic and ultramafic igneous rocks. Associated with Ca-plag,
Industrial / ecomonic uses:
Peridote (a translucent variety of olivine) is considered a 'gem' mineral. Also used as a refractory sand and infrequently as an abrasive.
Silicates
Orthoclase KAlSi
3
O
8
Hardness: 6 - 6.5
Density: 2.6
Habit: Polysynthetic twinning
Colours: Pinky-white
Crystal System: Orthorhombic
Cleavage: Perfect
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: None
Streak: White
Lustre: Vitreous
Identification in hand sample:
Colour and evidence of exsolution with plagioclase.
Associations:
Very common in granite, granodiorite, syenite and related felsic rocks.
Silicates
Quartz (crystal) SiO
2
Hardness: 7
Density: 2.7
Lustre: Vitreous / glassy
Habit: Crystalline
Colours: Clear transparent
Crystal System: Hexagonal
Cleavage: None
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Hardness, translucency and colour are indicators.
Silicates
Talc Mg
Hardness: 1
3
Si
Density: 2.6 - 2.8
4
O
10
Subclass: Phyllosilicate
(OH)
2
Streak: White
Lustre: Greasy
Habit: Compact masses, often no crystals.
Colours: White, or brownish, dark green, gray
Crystal System: Monoclinic
Cleavage: Perfect
Fracture: Uneven
Magnetism: None
Reaction with HCl: Unknown
Identification in hand sample:
Mineral is very soft and are often compact masses. Colour is typical and varies to brown, green or gray.
Silicates
Topaz Al
2
Hardness: 8
Density: 3.5
SiO
4
(F,OH)
2
Streak: White
Lustre: Glassy
Habit: Prismatic crystals
Colours: Almost brownish, any colour
Crystal System: Orthorhombic
Cleavage: Perfect
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Resembles quartz, but harder.
Associations:
Quartz
Industrial / ecomonic uses:
Gemstone
Silicates
Zircon (ZrSiO
4
)
Hardness: 7.5
Density: 4.6 - 4.7
Streak: White
Lustre: Subadamantine
Habit: Excellent tetragonal prisms
Colours: Colorless, red, brown, yellow, green or gray
Crystal System: Tetragonal
Cleavage: Imperfect
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: Unknown
Identification in hand sample:
Excellent crystalline habit, density and streak/colour are indicative.
Industrial / ecomonic uses:
Source of zirconium and hafnium
Carbonates
Azurite ---Cu
3
(CO
3
)
2
(OH)
2
Hardness: 3.5 – 4
Density: 3.8
Streak: Lighter blue
Lustre: Vitreous, earthy (fine grained)
Habit: Fine grained coating or fracture filling on rock
Colours: Azure blue
Crystal System: Monoclinic
Cleavage: Perfect
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: Strong
Identification in hand sample:
Colour is reliable, association with malachite is also indicative.
Associations:
Common mineral in the near-surface oxidized portion of copper-bearing hydrothermal sulfide mineral deposits. Associated with malachite.
Industrial / ecomonic uses:
A minor ore of copper, also has ornamental uses. Sometimes a pigment (when powdered).
Carbonates
Aragonite ----CaCO
3
Hardness: 3.5 – 4
Streak: White
Colours: Colourless or white
Lustre: Vitreous
Habit: Stalactitic aggregate of crystals or grains.
Crystal System: Orthorhombic
Cleavage: Pinacoidal
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: Strong
Identification in hand sample:
Stalactitic habit, hardness (greater than that of calcite) and colour.
Associations:
Occurs in carbonate-bearing blueschist metamorphic rocks. Associated with glaucophane, lawsonite, pumpellyite. Also forms at or near the surface in cave and hot spring deposits.
Industrial / ecomonic uses:
Same as calcite, however abundance is much much less than that of calcite, thus having little economic value.
Carbonates
Calcite (massive) CaCO
3
Hardness: 3 Density: 2.7
Streak: White Lustre: Vitreous
Habit: Fine grained aggregates (massive)
Colours: Colourless, white, gray, yellow-brown, pink, rose red
Crystal System: Hexagonal
Cleavage: Perfect rhombohedral
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: Strong
Identification in hand sample:
Recognized by hardness and cleavage. Dolomite and aragonite have higher densities.
Associations:
Common as a cementing agent in clastic sediments, or as fossil fragments. Essential constituent of limestone.
Industrial / ecomonic uses:
Used in the manufacture of portland cement (quicklime). Also used as a pharmaceutical (antacids, calcium supplements).
Carbonates
Calcite (sparry) (Iceland Spar) ---- CaCO
3
Hardness: 3 Streak: White
Density: 2.7
Lustre: Vitreous
Habit: Large crystals, some exhibiting common twin striae
Colours: White
Crystal System: Hexagonal
Cleavage: Perfect rhombohedral
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: Strong
Identification in hand sample:
Hardness, crystal habit and twin striae are typical.
Associations:
Common as a cementing agent in clastic sediments or as fossil fragments.
Industrial / ecomonic uses:
Used in the manufacture of portland cement (quicklime) and in the pharmaceutical industry.
Comments:
The transparent variety is called "Iceland Spar".
Carbonates
Dolomite ---- CaMg(CO
Hardness: 3.5 – 4
3
Streak: White
)
2
Density: 2.85
Lustre: Vitreous
Habit: Crystalline or aggregate, some with twin striae.
Colours: Pinky/peach, white, gray, brown.
Crystal System: Hexagonal
Cleavage: Perfect rhombohedral
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: Weak
Identification in hand sample:
Harder than calcite
Associations:
Major constituent of dolostone (aka dolomite).
Industrial / ecomonic uses:
Used in manufacture of portland cement.
Carbonates
Malachite ---Cu
Hardness: 3.5 – 4
2
(CO)
3
(OH)
Streak: Pale green
2
Density: 4.0
Lustre: Earthy
Habit: Very fine grained coating on other rocks, almost looks like paint.
Colours: Copper green
Crystal System: Monoclinic
Cleavage: Perfect
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: Strong
Identification in hand sample:
Colour is primary indicator, as well as habit.
Associations:
Common in near-surface oxidized portions of copper bearing hydrothermal sulfide mineral deposits.
Industrial / ecomonic uses:
Used as a minor ore of copper and also has ornamental uses due to vivid green colour.
Carbonates
Siderite ---- FeCO
3
Hardness: 3.5 – 4
Density: 4.0
Habit: Aggregate of crystals or grains
Colours: Tan brown (typical)
Streak: White
Lustre: Vitreous
Crystal System: Hexagonal
Cleavage: Perfect rhombohedral
Fracture: Uneven
Magnetism: None
Reaction with HCl: Weak
Identification in hand sample:
Identified by colour and hardness.
Associations:
Produced by hydrothermal alteration of limestone. May also occur as concretionary or oolitic forms in clay ironstones.
Industrial / ecomonic uses:
Sometimes used as an iron ore. Also used in pigments where a red or brown colour is desirable.
Halides
Fluorite --- CaF
2
Hardness: 4
Density: 3.1 - 3.3
Streak: White
Lustre: Vitreous
Habit: Crystals, usually cubic
Colours: Commonly colourless, blue, purple or green, but any is possible.
Crystal System: Isometric
Cleavage: Perfect octahedral
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: Unknown
Identification in hand sample:
Cubic crystals, hardness and colour indicative.
Associations:
Common in hydrothermal mineral deposits associated with sulfides, carbonates and barite.
Industrial / ecomonic uses:
Main source of fluorine (which is added to drinking water, toothpaste, used as a flux in industrial uses and as part of CFCs)
Halides
Halite (Salt) --- NaCl
Hardness: 2
Density: 2.1 - 2.2
Streak: White
Lustre: Waxy
Habit: Crystalline (cubic)
Colours: Colourless or white if pure, any colour if not
Crystal System: Isometric
Cleavage: Cubic
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Has a salty taste. Cubic cleavage and hardness are indicative for the less brave geologists.
Associations:
Abundant in marine evaporite deposits and may form beds hundreds to over a thousand meters thick. Associated minerals include calcite, dolomite, gypsum, anhydrite and sylvite.
Hydroxide
Limonite-- FeO(OH)
Hardness: 5 - 5.5
Density: 2.7 - 4.3
Streak: Yellow-brown
Lustre: Earthy
Habit: Fine grained aggregate, powdery coating.
Colours: Orange, yellow-brown
Crystal System: Unknown
Cleavage: None
Fracture: Uneven
Magnetism: None
Reaction with HCl: Very weak
Identification in hand sample:
Colour is best indicator, followed by habit.
Associations:
Common weathering product of iron-rich rocks.
Comments:
The fine brownish coloured mineral is usually weathered goethite.
Oxides
Chromite (FeCr
2
Hardness: 5.5
O
4
)
Density: 4.5 - 4.8
Streak: Brown
Lustre: Metallic to pitchy
Habit: Fine grained aggregate.
Colours: Black
Crystal System: Isometric
Cleavage: None
Fracture: Uneven
Magnetism: Weak
Reaction with HCl: Unknown
Identification in hand sample:
Resembles magnetite and ilmenite, but not very magnetic and has a more resinous lustre.
Associations:
Found in mafic and ultramafic igneous rocks such as gabbro, peridotite, dunite and pyroxenite as an accessory mineral.
Industrial / ecomonic uses:
Only ore mineral for chromium.
Oxides
Corundum (Al
2
Hardness: 9
O
3
)
Density: 4.0 - 4.1
Habit: Well-formed crystals
Streak: White
Lustre: Vitreous to adamantine
Colours: Any. White, gray or gray-blue, or red (ruby), blue (sapphire), yellow, green.
Crystal System: Hexagonal
Cleavage: None
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Hardness is best indicator, crystal habit is good too.
Associations:
Gem quality corundum comes from metamorphed limestone or dolostone (needs high Al concentrations, here presumably from detrital deposits)
Industrial / ecomonic uses:
Used as a gemstone. High-quality ruby can be more valuable than diamond. Corundum is also used as an abrasive in sandpaper, polishing compounds, etc.
Oxides
Hematite (massive) -- (Fe
2
O
3
)
Hardness: 5 – 6
Density: 5.3
Streak: Deep red
Lustre: Earthy
Habit: Very fine grained aggregate of red crystals.
Colours: Deep red
Crystal System: Hexagonal
Cleavage: None
Fracture: Subconchoidal
Magnetism: Weak
Reaction with HCl: Unknown
Identification in hand sample:
Deep red streak and earthy lustre are indicative. SG may also be indicative.
Associations:
Produced by weathering or hydrothermal alteration of iron-bearing minerals. May be found in some syenite, trachyte, granite and rhyolite.
Industrial / economic uses:
Important ore or iron, sometimes used as a gemstone.
Comments:
May become magnetic when heated.
Oxides
Hematite (specular) (Fe
Hardness: 5 – 6
Density: 5.3
Streak: Deep red
Lustre: Metallic
Habit: Micaceous/tabular habit.
Colours: Steel gray
2
O
3
)
Crystal System: Hexagonal
Cleavage: None
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: Unknown
Identification in hand sample:
Shining gray metallic lustre and deep-red streak are indicative.
Associations:
Produced by weathering and hydrothermal alteration of iron-bearing minerals.
Industrial / ecomonic uses:
Important ore of iron.
Oxides
Magnetite (Fe
3
Hardness: 5.5 - 6.5
O
4
)
Density: 5.2
Streak: Black
Lustre: Dull metallic to splendent
Habit: Granular habit of fine grains. Some octahedral crystals may form.
Colours: Black
Crystal System: Isometric
Cleavage: None
Fracture: Subconchoidal
Magnetism: Strong
Reaction with HCl: Unknown
Identification in hand sample:
Highly magnetic. Habit and colour are also indicators.
Associations:
Very common accessory mineral found in a wide variety of igneous and metamorphic rocks, usually as small grains.
Industrial / ecomonic uses:
Mined for iron. Crushed magnetite also been used as aggregate to make high-density concrete for specialized applications, such as nuclear reactors.
Oxides
Pyrolusite (MnO
Hardness: 2 - 6.5
2
)
Streak: Black
Density: 5.1
Lustre: Earthy
Habit: Massive, compact, columnar or fibrous. Prismatic crystals are rare. Sometimes forms as dendritic coatings on other rocks.
Colours: Black
Crystal System: Tetragonal
Cleavage: Perfect
Fracture: Uneven
Magnetism: None
Reaction with HCl: Very weak
Identification in hand sample:
Will leave sooty marks if touched.
Associations:
Found in bog and marine deposits
Industrial / ecomonic uses:
Manganese is used to colour bricks.
Phosphates
Apatite Ca
5
(PO
4
)
3
( OH,F,Cl)
Hardness: 5
Density: 3.1 - 3.2
Streak: White
Lustre: Vitreous, glassy
Habit: Massive
Colours: Grayish blue-green, or any.
Crystal System: Hexagonal
Cleavage: Poor
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: Weak
Identification in hand sample:
Colour, crystal habit and hardness are good indicators.
Associations:
Common accessory mineral in all environments.
Industrial / ecomonic uses:
Source of phosphate used in fertilizers and other industrial applications. Fluorine is also extractable from apatite.
Comments:
Same stuff as in bones and teeth.
Sulfates
Alabaster (Gypsum) CaSO
4
Hardness: 2 Streak: White
Density: 2.3
Lustre: Vitreous
Habit: Granular / massive form of gypsum
Colours: Colourless or white (typical)
Crystal System: Monoclinic
Cleavage: Perfect
Fracture: Splintery
Magnetism: None
Reaction with HCl: Weak
-
2
H
2
O
Identification in hand sample:
Identified by hardness and good cleavage.
Associations:
Common in marine evaporite deposits.
Industrial / ecomonic uses:
Modern use is in gypsum wallboard used in construction. Used as an ornamental stone and for sculpture due to its softness.
Sulfates
Barite (BaSO
Hardness: 3 - 3.5
4
)
Streak: White
Density: 4.5
Lustre: Vitreous
Habit: Fine grained aggregates
Colours: Colourless, white, gray, yellowish, brown, reddish, bluish or greenish
Crystal System: Orthorhombic
Cleavage: Perfect
Fracture: Uneven
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
High specific gravity is best indicator, colour and fracture are also helpful.
Associations:
Common in hydrothermal veins, associated with galena, sphalerite, pyrite, quartz, fluorite and carbonates.
Industrial / ecomonic uses:
Primary ore for barium, also used as an additive to drilling mud (because of high SG). Has over 2000 uses in industry.
Sulfates
Selenite (Gypsum) CaSO
4
Hardness: 2
Density: 2.3
Streak: White
Lustre: Vitreous
Habit: Large crystalline habit
Colours: Colourless, clear
-
Crystal System: Monoclinic
Cleavage: Perfect
Fracture: Splintery
Magnetism: None
Reaction with HCl: Weak
2
H
2
O
Identification in hand sample:
Single large crystals of gypsum, typified by hardness and transparency.
Associations:
Found in marine evaporites.
Industrial / ecomonic uses:
Used in gypsum wallboard.
Sulfides
Chalcocite (Cu
2
Hardness: 2.5 – 3
Density: 5.5 - 5.8
S)
Streak: Blackish lead-gray
Lustre: Dull metallic
Habit: Chunky fracture, massive habit.
Colours: Blackish lead-gray
Crystal System: Monoclinic
Cleavage: Indistinct
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: None
Identification in hand sample:
Black colour with sooty appearance on weathered surfaces and sectile character are distinctive. Bronze-yellow on fresh surfaces.
Associations:
Found in the supergene-enriched zone of copper-bearing hydrothermal sulfide deposits.
Industrial / ecomonic uses:
Mined as a source of copper.
Sulfides
Chalcopyrite (CuFeS
Hardness: 3.5 – 4
2
)
Streak: Greenish black
Density: 4.3 - 4.4
Lustre: Metallic
Habit: Fine grained to massive aggregates.
Colours: Brass yellow, may be tarnished and iridescent.
Crystal System: Tetragonal
Cleavage: Poor
Fracture: Conchoidal
Magnetism: None
Reaction with HCl: Very weak
Identification in hand sample:
Distinctive brassy-green colour. Has a richer yellow colour than pyrite and a lower hardness.
Associations:
Most common copper-bearing mineral, found in many hydrothermal sulfide deposits.
Often associated with galena, sphalerite, pyrite and other sulfides.
Industrial / ecomonic uses:
Mined for its copper which has innumerable industrial uses.
Sulfides
Galena (PbS)
Hardness: 2.5
Density: 7.6
Streak: Lead gray
Lustre: Metallic
Habit: Excellent cubic cleavage, crystals usually very evident
Colours: Lead gray
Crystal System: Isometric
Cleavage: Perfect cubic
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: Weak
Identification in hand sample:
Gives off a rotten-egg smell in HCl, very high SG, very metallic lustre.
Associations:
Very common in hydrothermal sulfide deposits. Associated with sphalerite, pyrite, chalcopyrite, quartz, calcite, fluorite, and/or barite.
Industrial / ecomonic uses:
Main ore from which lead is extracted, also a source of silver.
Sulfides
Pyrite (crystalline) FeS
2
Hardness: 6 - 6.5
Density: 5.0
Streak: Greenish/brownish black
Colours: Bronze-yellow
Crystal System: Isometric
Cleavage: Good
Fracture: Conchoidal
Magnetism: Unknown
Reaction with HCl: None
Lustre: Metallic
Habit: Perfect cubic crystalline habit.
Identification in hand sample:
Colour, hardness, streak and habit are best indicators. Gives off sparks when struck with a hard metal object.
Associations:
Most common sulfide mineral, almost always present in hydrothermal deposits.
Industrial / ecomonic uses:
May be used as source of iron or sulfur, but not normally of economic value.
Sulfides
Stibnite (Sb
2
Hardness: 2
S
3
)
Density: 4.6 - 4.7
Streak: Lead gray
Lustre: Metallic
Habit: Range from fine to medium grains to prismatic crystals (with longitudinal striations).
Complex terminations, radiating acicular groups.
Colours: Lead gray, black, iridescent tarnish
Crystal System: Orthorhombic
Cleavage: Perfect
Fracture: Subconchoidal
Magnetism: None
Reaction with HCl: Weak
Identification in hand sample:
Colour and small subhedral crystals are indicators. Also rather soft.
Associations:
Forms in low-temperature hydrothermal veins, or is deposited from hot mineral springs.
Industrial / ecomonic uses:
Important source of antimony (Sb)
Native Elements
Copper (Cu)
Hardness: 2.5 - 3
Density: 8.9
Streak: Same
Lustre: Metallic
Habit: Massive, platey or dendritic habit.
Colours: Light rose on fresh surface, copper-red on tarnished surface
Crystal System: Isometric
Cleavage: None
Fracture: Hackly
Magnetism: None
Reaction with HCl: Weak
Identification in hand sample:
Colour and metallic characteristics are diagnostic.
Associations:
Found associated with mafic volcanic rocks, formed by reaction between Cu-bearing solutions and Febearing minerals. Associated minerals are cuprite, chalcocite, bornite, epidote, calcite, chlorite and zeolites.
Industrial / ecomonic uses:
Used as electrical wire due to electrical conductivity and relatively low price.
Comments:
Obviously a source of copper, however most copper in modern times is mined from sulfide minerals.
Native Elements
Graphite (C)
Hardness: 1 – 2
Density: 2.1 - 2.3
Habit: Fine grained aggregate
Colours: Silver-black
Crystal System: Hexagonal
Cleavage: Perfect basal
Fracture: Uneven
Magnetism: None
Reaction with HCl: None
Streak: Black
Lustre: Dull metallic, greasy feel
Identification in hand sample:
Softness and lustre are best indicators.
Associations:
Common in pelitic metamorphic rocks such as phyllite, slate and schist. Produced as a result of decomposition of organic material.
Industrial / ecomonic uses:
Used in pencil leads and as a dry lubricant.
Comments:
Due to its softness, graphite is usually quite messy!
Reference