Exercise C2 Melting Point & E1 Refractive Index
advertisement

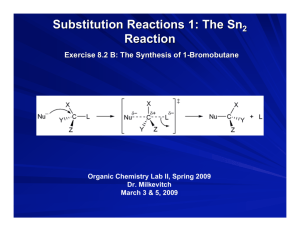
Substitution Reactions 1: The Sn2
Reaction
The Synthesis of 1-Bromobutane
Organic Chemistry Lab II, Fall 2009
Dr. Milkevitch
November 9 & 12, 2009
Substitution Reactions
Today: Learn about an Sn2 reaction
Type of reaction: Substitution
One thing substitutes for another
Primarily a reaction of alkyl halides
The Sn2 Reaction
Sn2 = Substitution, nucleophilic,
bimolecular
Substitution: One things substitutes for
another
Nucleophilic: Nucleophile does the substituting
– Something must leave
Called the leaving group
Bimolecular: The kinetics of the reaction
involve the concentrations of 2 reactants
Mechanism of the Sn2 Reaction
General mechanism:
substrate
Nu
L
_
= nucleophile (“nucleus loving”), species that seeks a + charge
= leaving group, the species that leaves
More Detail
X
X
_
substrate
L
Nu
Y
X
Nu
L
L
Y
Z
X
Nu
+L
Y
Z
Y
Z
Transition state
Z
Nucleophile
substitutes for
L group
Factors That Affect Sn2
Reactions
Strength of nucleophile
Structure of substrate
Nature of solvent
Concentration of reactants
Nature of the leaving group
Kinetics of the Sn2 Reaction
Reaction kinetics: how fast a reaction goes
Appearance of product per unit time
Rate Law:
– Rate = k{A}x{B}y
Reaction rate dependent on concentrations
of reactants
First order reaction: rate dependent on the concentration
of one reactant
Second order reaction: rate dependent on the
concentration of both reactants
Kinetics of the Sn2 Reaction
“2” means the kinetics are second
order
Rate of reaction dependent on
concentration of both reactants
Double concentration of either one, rate
doubles
Synthesis of 1-bromobutane from 1butanol
Acid catalyzed
reaction
Mechanism
H2SO4 + NaBr
HBr + NaHSO4
Br
Good leaving
group
++HH2O
Reflux
Reflux: continual boiling
of a solution in a vial or flask
where solvent is continually
returned to the reaction vessel
from a condenser atop the vial
or flask
Water cooled condenser is
used
Possible to heat a reaction at
the boiling point of the
solvent for extended periods
Procedure
Weigh out 12.5 g of NaBr and add it to 12 ml of ddH2O in a 100 ml RB flask (with
stir bar)
Clamp to ring stand, stir until NaBr dissolves
Add 10 ml of 1-butanol to this flask
Place in an ice bath and continue to stir
Measure out 11 ml of concentrated H2SO4 into a clean 50 ml erlenmeyer flask
Put this in its own ice bath
Carefully add the acid to the solution of NaBr and 1-butanol in small amounts
(maybe 0.5 ml) with stirring. Both solutions must be in ice baths.
Once completed, remove the RB flask (with acid, 1-butanol in it) and fit a
heating mantle with condenser.
Reflux for 45 min with stirring.
When done refluxing, remove the condenser and fit a still head for distillation.
Using a 50 ml RB flask for collection, distill until 20-25 ml of distillate has been
collected.
You should have 2 layers in the receiving flask.
Parafilm the receiving flask and leave it until next week. We will complete the
experiment then.
Acid Addition
Set up your solution of NaBr/H2O/1-butanol
for acid addition as follows:
RB flask
50 ml erlenmeyer flask
with acid, in an ice bath
Crystalling
Dish w/ ice
Stir plate
Your Report
This is a 2 week experiment
– This week: the synthesis
– Next week: purification & characterization
Substitution Reactions 1: The Sn2
Reaction
The Synthesis of 1-Bromobutane
Part II: Workup
Organic Chemistry Lab II, Spring 2009
Dr. Milkevitch
November 16 & 18, 2009
Procedure II
Remove parafilm, pour your distillate (solution in RB flask) into a 125
ml separatory funnel
Add 50 ml of ddH2O to the separatory funnel, stopper and shake like
you did in the extraction lab
Allow layers to separate, draw off bottom organic layer into a 125 ml
erlenmeyer flask
Pour off the upper (aqueous) layer and set aside (do NOT throw away
yet)
Transfer the organic layer back into the separatory funnel and add 25
ml of H2O
Stopper and shake the separatory funnel again, let layers separate
Draw off the lower organic layer again, into a 125 ml erlenmeyer flask
Pour off the upper (aqueous) layer and set aside (again do NOT throw
it away yet)
Transfer the organic layer back into the separatory funnel, add 25 ml
of saturated sodium bicarbonate solution (the bicarb solution is in the
hood)
Procedure III
Swirl the separatory funnel, notice CO2 escaping. Carefully
shake the separatory funnel with frequent venting
Allow the layers to separate
Draw off the bottom organic layer into a 125 ml erlenmeyer
flask
Pour off the upper water layer and set aside (again, do NOT
throw away yet)
Filter the organic layer through a layer of anhydrous
magnesium sulfate.
Collect the dried organic layer
Place the dried organic layer in a pre-weighed 50 ml RB flask
with a spin bar
Distill the organic layer until most of the liquid in the distilling
flask has distilled over (about 105 deg C)
Weigh the receiving flask, determine weight of product
Characterization
Analyze a sample of your product by GC
Analyze by TLC :
–
–
–
–
Spot standards: 1-bromobutane, 1-butanol, dibutyl ether
Spot your distilled product also
Mobile phase: ethyl acetate
Analyze under UV light in UV light box
If you have enough product, acquire an IR
spectrum:
– Consult with Dr. M.
Complete reaction worksheet