PowerPoint - pH of Salt Solution Prediction - Ka, Kb
advertisement

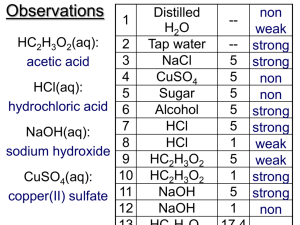
Predicting the pH of salt solutions Hydrolysis of ions Hydrolysis refers to a reaction with water (e.g. splitting water into H+ and OH–) When salts are added to water, pH can change E.g. when Na3PO4 is added to water, ions form Na3PO4(aq) 3Na+(aq) + PO43–(aq) These ions may react with H2O, affecting the pH PO43–(aq) + H+(aq) HPO42–(aq) Na+(aq) + OH–(aq) NaOH (aq) If the anion (-ve) reacts to remove lots of H+ but the cation (+ve) removes very little OH–, then H+ will decrease and the solution will be basic. The degree of hydrolysis • • • • • PO43–(aq) + H+(aq) HPO42–(aq) Na+ + OH– NaOH The problem with writing equilibria this way is we do not know the strength of the reactions However, if we reverse the reaction we can look up Ka and Kb values (pg. 608, 615): –13 2– 3– + Ka= 4.5 x 10 HPO4 PO4 + H Kb= 55 NaOH Na+ + OH– Small Ka: few products; adding PO43– = shift left Large Kb: mostly products; Na+ has little affect Thus, adding Na3PO4 will cause more H+ to be removed, resulting in a basic solution Accuracy of predictions Theoretically, using Ka and Kb values you could predict the exact pH resulting from a certain salt being added to distilled water. However, you only need to be able to predict if a solution will be acidic, basic, or neutral. Note: you can’t judge the pH change solely on the difference between Ka and Kb. Other factors are involved (e.g. the formula of the compound and its molar mass both affect [ ]) Note: hydrolysis refers to reactions with water. Several variations for writing equilibriums exist. However, focusing on how the H+/OH– balance of water is affected is easiest. Steps in determining pH 1. Write the ions that form: e.g. NH4CN NH4+ + CN – 2. Determine the reaction ions have with water: NH4++OH– NH3 + H2O, NH3 + H2O NH4+ + OH– CN – + H+ HCN, HCN CN– + H+ 3. Look up the Ka of the conjugate acid and the –][H+] +][OH–]base: [CN Kb of the[NH conjugate 4 Ka = Kb = [HCN] [NH3] –10 –5 = 6.2 x 10 = 1.8 x 10 4. Determine if more H+ or OH– is removed: More H+ is removed, therefore BASIC Buffers - lab Read 15.6 (621-623) up to and including special topic 15.2 (carbonate buffer) Calibrate pH meter, get a plastic bottle with distilled H2O to rinse your pH meter btw tests You will use 4 solutions (20 mL of each): distilled water, water + NaC2H3O2 (5 scoops), 0.2 M HC2H3O2, 0.2 M HC2H3O2 + NaC2H3O2 For each, record the initial pH and the pH upon addition of 5, 10, and 15 drops of 1 M HCl Remake the 4 solutions For each, record the initial pH and the pH upon addition of 5, 10, and 15 drops of 1 M NaOH HCl 0 5 10 15 Na OH 0 5 10 15 H2O H 2O NaC2H3O2 HC2H3O2 NaC2H3O2 + HC2H3O2 NaC2H3O2 HC2H3O2 NaC2H3O2 + HC2H3O2 HCl H2O 0 5 10 15 Na OH 0 5 10 15 6.9 1.9 1.7 1.6 H 2O 6.9 11.1 11.3 11.4 NaC2H3O2 HC2H3O2 8.0 6.3 5.6 5.7 2.8 2.6 2.2 2.0 NaC2H3O2 HC2H3O2 7.7 10.3 10.5 10.8 2.6 2.7 3.3 3.4 NaC2H3O2 + HC2H3O2 5.1 5.0 5.0 4.9 NaC2H3O2 + HC2H3O2 5.3 5.2 5.2 5.3 Buffers - summary Solutions with buffers resist changes in pH, when small amounts of acid or base are added Buffers are important in blood, cells, resisting the effects of acid rain on lake ecosystems. A buffer is created when a weak acid is mixed with a salt that contains the identical ion. Two equilibria contribute to the consistent [H+] HA H+ + A– Na+ + A– NaA For more lessons, visit www.chalkbored.com