Unit 1 - Intro to Sc..
advertisement

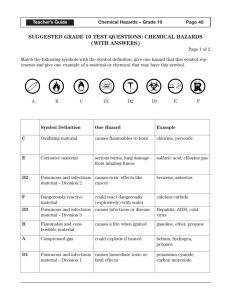
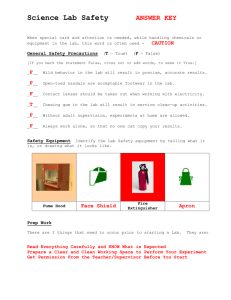
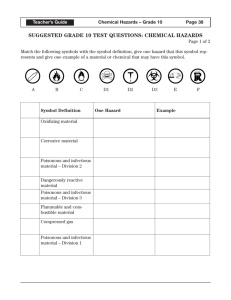
An Introduction to Basic Skills and Procedures 1) Lab Equipment Refer to Handouts – Laboratory Equipment Handout 2) Lab Safety a) General Rules – Fires – Splashes – Broken Glass 2) Lab Safety 2) Lab Safety 2) Lab Safety b) Safety Equipment What are 5 pieces of Safety Equipment in a Science Classroom? 1) Eye Wash – used to wash harmful chemicals from your eyes 2) First Aid Kit – used to help treat minor injuries 3) Water Taps – Can be used to wash harmful chemicals - can also be used to extinguish fires 4) Fire Extinguisher – used to put out fires. 5) Fume Hood – used to remove harmful fumes from the area. These are usually vented directly outside. WHMIS Stands for “Workplace Hazardous Materials Information System.” Consists of 3 Main Parts. A) Labels 1) Supplier Labels – Are provided by the companies that produce and distribute the chemicals – Contain information about the product and safety instructions for safe handling. – They also use safety symbols 2) Workplace Labels – are used by working environments (such as schools that have chemicals in them). B. MSDS Stands for “Material Safety Data Sheets.” Provides detailed information about the chemicals being used such as: melting point, boiling point, physical and chemical characteristics, possible reactions, risk phrases, safe-handling procedures, etc. C. Symbols There are: • 8 Symbols • 6 Classes Class D has 3 Divisions What substances might belong in each category? What risks and cautions might be associated with each category? C. Symbols CLASS A: COMPRESSED GAS This class includes compressed gases, dissolved gases, and gases liquefied by compression or refrigeration. CLASS B: FLAMMABLE AND COMBUSTIBLE MATERIAL This class includes solids, liquids, and gases capable of catching fire in the presence of a spark or open flame under normal working conditions. CLASS C: OXIDIZING MATERIAL These materials increase the risk of fire if they come in contact with flammable or combustible materials. C. Symbols CLASS D 1 : POISONOUS AND INFECTIOUS MATERIAL Materials Causing Immediate and Serious Toxic Effects These materials can cause death or immediate injury when a person is exposed to small amounts. Examples: sodium cyanide, hydrogen sulphide CLASS D 2 : POISONOUS AND INFECTIOUS MATERIAL Materials Causing Other Toxic EFFECTS These materials can cause life-threatening and serious long-term health problems as well as less severe but immediate reactions in a person who is repeatedly exposed to small amounts. CLASS D 3 : POISONOUS AND INFECTIOUS MATERIAL Biohazardous Infectious MATERIAL These materials contain an organism that has been shown to cause disease or to be a probable cause of disease in persons or animals. C. Symbols CLASS E: CORROSIVE MATERIAL This class includes caustic and acid materials that can destroy the skin or eat through metals. Examples: sodium hydroxide, hydrochloric acid, nitric acid CLASS F: DANGEROUSLY REACTIVE MATERIAL These products may self-react dangerously (for example, they may explode) upon standing or when exposed to physical shock or to increased pressure or temperature, or they emit toxic gases when exposed to water. Graphing Rules 1) Identify the independent and the dependent variables. a) Independent is the variable that the experimenter changes (usually time) and is always plotted on the x-axis. b) Dependent variable is the variable that changes as a result of the experiment and is always plotted on the y-axis. 2) Label each axis with the name of the variable and the unit. 3) Title your graph (ex. A Graph of Dependent vs. Independent) 4) Include your name, date, class, and period on the top right hand corner of your graph. 5) Choose your scale carefully and make your graph as large as possible. 6) It is NOT necessary that the graph always go through the origin. 7) If required, calculate the slope on the graph. 8) Make sure to use a best fit line if the graph is linear. Use a smooth (free-hand) curve if the graph is a curved one. A Graph of Speed Vs. Time Speed (m/s) 21 18 15 12 9 6 3 0 0 2 4 6 Time (s) 8 10 12 The Metric System The metric system conveniently uses the number ten as a conversion factor. The Metric System Commonly used base units: Length: metre (m) Weight: gram (g) Volume: litre (l) M k h da d BASE c m ų The Metric System To convert, ask yourself … 1.Which unit is larger? 2.Will my # be larger or smaller? 3.How many places do I move the decimal? M k h da d BASE c m ų M k h da d c m BASE Examples: 1) 2) 3) 4) 5) 100cm = ____________ dm 53kL = ____________ daL 0.025MA = __________ hA 50m = ____________ cm 0.000046mΩ = __________ųΩ ų M k h da d c m ų BASE Examples: 6) 600 W = _____________ kW 7) 200 mm = _____________ m 8) 0.0072 ML = ____________ hL 9) 45.3 mL = _____________ L 10) 0.00096 mW = ___________ ųW Lab Write Up Requirements 1) Title Page (separate or at top of page) – include the name of the lab, your name, your partner’s name, the date the lab is due, and the class that you are in. 2) Purpose or Objective – this is either given or determined by the student. 3) Hypothesis – is an educated prediction made before the lab. It is okay to be wrong! 4) Materials – a list of all the equipment used in the lab. You may be required to draw a diagram of the set up. 5) Procedure – detailed step-by-step directions of what you did in your lab should be written here. 6) Data and Observations – consists of all measurements, observations, and calculations. Usually in the form of a table or chart. This is where a graph would go. 7) Analysis/Questions – All questions asked will be answered in this section. 8) Conclusion – should relate back to the purpose of the lab. What were your results? Did the experiment agree with your hypothesis? 9) Sources of Error – Record two external factors that could have effected the result of your experiment. Physical vs. Chemical Change • physical change - a change in matter in which no new type of matter is produced; for example, freezing, melting and boiling. • chemical change - a change in which one or more new substances are produced with properties different from those of the starting substance. Quantitative vs. Qualitative Observations • Quantitative - data that consists of numbers and/or units of measurements; obtained through measurement and mathematical calculations (length, width, height, area, volume, perimeter, mass, density, circumference). • Qualitative - information gathered in which no measurement takes place, instead the five senses are used to make the observation (see, hear, taste, feel, smell).