Potential and Current Flow: non-Faradaic
advertisement

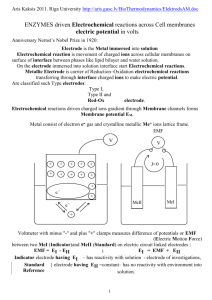
University of Pittsburgh Cathedral of Learning America’s Smartest City by Movoto Blog Rated Most Livable City by Places Rated Almanac and The Economist Named among Best in the World Places to Visit by National Geographic Traveler Basic Introduction to Electrochemical Cells and Methods David H. Waldeck Department of Chemistry University of Pittsburgh : http://www.chem.pitt.edu/ The Electrochemical Cell The chemical An reaction electrochemical cell is a device that transduces energy 2 AgI (s) + Pb → 2 Agforms. (s) + PbI2 (s) between chemical and(s) electrical consists of a reduction reaction and an oxidation reaction An electrochemical cell has at least two electrodes and an electrolyte, as such both ion and electron transport are important to consider. The Electrochemical Cell The chemical reaction 2 AgI (s) + Pb (s) → 2 Ag (s) + PbI2 (s) consists of a reduction reaction and an oxidation reaction A reduction, which occurs at the cathode AgI (s) + e- → Ag (s) + I- (aq) E0 AgI/Ag = -0.1522 V Absolute temperature Molar gas constant EAgI/Ag= E0 AgI/Ag − activity =1 𝑎𝐴𝑔 𝑎𝐼− 𝑅𝑇 ln 1𝐹 𝑎𝐴𝑔𝐼 activity ≠ 0 activity =1 standard potential Faraday’s constant # of electrons transferred in the reaction The Electrochemical Cell The chemical reaction 2 AgI (s) + Pb (s) → 2 Ag (s) + PbI2 (s) consists of a reduction reaction and an oxidation reaction … and an oxidation which occurs at the anode Pb (s) + 2 I- (aq) → PbI2(s) + 2e- E0 Pb/PbI2 = 0.365 V activity =1 EPb/PbI2= E0 Pb/PbI2 − 𝑅𝑇 𝑎𝑃𝑏𝐼2 ln 2𝐹 𝑎𝑃𝑏 𝑎𝐼− 2 activity ≠ 0 activity =1 standard potential # of electrons transferred in the reaction The Electrochemical Cell Hence, we find that Ag I (aq) + e- → Ag (s) + I- (aq) E0 AgI/Ag = -0.152 V Pb (s) + 2 I- (aq) → PbI2(s) + 2 e- E0 Pb/PbI2 = 0.365 V E0 cell = 0.213V 2AgI (s) + Pb (s) → 2Ag (s) + PbI2 (s) E = EAgI/Ag + EPb/PbI2 = E0 AgI/Ag +E0 𝑎𝐴𝑔 𝑎𝐼− 𝑅𝑇 𝑅𝑇 𝑎𝑃𝑏𝐼2 ln − ln Pb/PbI2− 1𝐹 𝑎𝐴𝑔𝐼 2𝐹 𝑎𝑃𝑏 𝑎𝐼− 2 = E0 AgI/Ag + E0 Pb/PbI2 − 𝑅𝑇 ln 𝐹 𝑎𝐼− + 𝑅𝑇 ln 2𝐹 = E0 AgI/Ag + E0 Pb/PbI2− = E0 cell = 0.213V 𝑎𝐼− 2 The Electrochemical Cell ϕ = E = 0.213 V Small changes in the applied potential allows us to reverse the direction of the chemical reaction. A galvanic cell; i.e., chemical reaction does electrical work. Electrolytic cell; i.e., electrical work drives chemical reaction. The Electrochemical Cell The connection between the electrochemical potential and G. ϕ = E = 0.213 V The reversible work done by the system is -wrev = E∙I∙t + PΔV and it is related to the Gibbs energy at constant T and P, namely ΔG = wrev + PΔV = - E∙I∙t = - E∙Qtotal = - E∙n∙F or ΔrG = ΔG/n = - E∙F The cell’s EMF is a direct measure of the Gibbs energy for the reaction. The Electrochemical Cell ϕ = E = 0.213 V Because ΔrG = - E∙F we can measure the temperature dependence of the EMF and find the molar entropy Δ𝑟 𝑆 = − 𝜕Δ𝑟 𝐺 𝜕𝐸 =𝐹 𝜕𝑇 𝑃 𝜕𝑇 𝑃 , DS ~14.5 J/(mol-K) and thus we also have the molar enthalpy, via ΔrH = ΔrG + T ΔrS = - E∙F + 𝐹 𝜕𝐸 𝜕𝑇 𝑃 Reference Electrodes &Electrode Potential We can use a standard half cell reaction such as 2 H+ (aq) + 2e- → H2 (g) E0 H+/H2 and measure the potentials of other half cell reactions, such as Cu2+ (aq) + 2 e- → Cu(s) E0 Cu2+/Cu with respect to it. For the electrochemical cell reaction H2(g) + Cu2+ (aq) → Cu (s) + 2H+ (aq) E0 cell Under standard state conditions (all activities equal to one), we find that E0 cell =E0 Cu2+/Cu - E0 H+/H2 = 0.345 V. If we define E0 H+/H2 = 0.0 V, then E0 Cu2+/Cu = 0.345 V NHE is commonly used to define the zero of the electrochemical potential scale. Reference Electrodes & Electrode Potential More common reference electrodes are AgCl (s) + e- → Ag (s) + Cl- (aq) 𝑅𝑇 EAgCl = E0 𝐴𝑔/𝐴𝑔𝐶𝑙 - 𝐹 𝑙𝑛 𝐶𝐶𝑙− 𝐾𝐴𝑔𝐶𝑙 For a saturated KCl solution EAgCl = 197 mV at 298 K Hg22+ + 2e- → 2 Hg Ecalomel = E0 + 𝑅𝑇 𝐹 𝑙𝑛 𝐶𝐶𝑙− 𝐾𝑠 For a saturated KCl solution ESCE = 241.2 mV at 298 K The Absolute Electrode Potential Relate the half cell reaction: 2 H+ (aq) + 2e- → H2 (g) with E0 H+/H2 to the vacuum potential, by using a thermodynamic cycle. 0 0 0 E0 H2/H+= ∆𝑟 𝐺 0 - 𝐹 = 1 ∆𝑑𝑖𝑠𝑠 𝐺 0 𝐻2 −𝐹 2 + 𝑁𝐴 ∙ 𝐸𝑖𝑜𝑛 𝐻 + ∆𝑠𝑜𝑙𝑣 𝐺 0 𝐻 + + 𝑁𝐴 ∙𝑊 𝑃𝑡 𝐹 so that E0 H2/H depends on intrinsic properties of the redox couple and the electrode material. The Absolute Electrode Potential Define E0 1 ∆𝑑𝑖𝑠𝑠 𝐺 0 𝐻2 + 𝑁𝐴 ∙ abs(H2/H+)=− 𝐹 2 𝑁 ∙𝑊 𝑃𝑡 = E0(H2/H+) - 𝐴 𝐹 𝐸𝑖𝑜𝑛 𝐻 + ∆𝑠𝑜𝑙𝑣 𝐺 0 𝐻+ Using experiment, workers have related the half-cell potential to the vacuum potential (e.g., measure work function Pt in contact with solution (values range from 4.4 to 4.8 V --- IUPAC recommends 4.44 ± 0.02 V. Thus E0 abs(H2/H+) = E0(H2/H+) - 4.44 𝑉 = −4.44 𝑉 Comments • about 1.21 V below W(Pt) measured in vacuum • use to find ∆𝑠𝑜𝑙𝑣 𝐺 0 𝐻+ =-1102.4 kJ/mol (excellent agreement with -1104.5 kJ/mol as found from cluster ion data) • For a half-cell reaction, M+ + e- M, we find that E0 abs(M+/M) = E0(M+/M) -E0 abs(H2/H+) = E0(M+/M) + 4.44 𝑉 Potentiometry: Equilibrium Measurements No current flows and system at equilibrium. Potential provides information on • Gibbs energy, entropy, etc. • Nernst Equation • Activities of ions, such as pH, etc. • Concentration cells • Activity coefficients and solution thermodynamics • Equilibrium constants • Titrations • Solubility products • Fuel cell and battery energetics Kinetics through Electrochemical Measurements Apply perturbation and measure response: Voltammetry example: Apply a potential jump and measure a current response. Issues affecting Meaningful Measurements The 2 electrode cell: iRCurrent drop: can affect Reference Electrode Potential Theexample, current flow through thethe solution causes a voltage drop soreference that the applied For at high currents Cl- concentration of Ag/AgCl electrode potential between the working and reference electrode is not the true potential could change and affect E 𝑅𝑇 𝐶 drop … EAgCl = E0 𝐴𝑔/𝐴𝑔𝐶𝑙 - 𝑙𝑛 𝐶𝑙− 𝐹 𝐾𝐴𝑔𝐶𝑙 Issues Affecting Meaningful Measurements Ohmic losses (iR drop) The resistive loss in the solution causes a change in the potential and can affect the measurement. current source Electron current, Ie, is flowing in the metal wires, while ion current, Iion, is flowing in the cell. In total Iion=Ie A Potentiostatic Cell Can Resolve these Issues Use a 3-electrode cell The reference electrode measures potential and has little current flow. Most of the current goes between working and auxiliary electrode A Potentiostatic Cell Can Resolve these Issues iRs Drop becomes an iRu drop In this way the potential drop is minimizes if the reference is placed close to working. Potential and Current Flow: non-Faradaic Ideal Polarized Electrode -- An electrode in which no charge transfer occurs as the potential is changed. Some electrodes approximate over limited ranges: • Hg electrode over 2V range in KCl solution • Hg oxidation at +0.25 V versus NHE • K+ reduction at -2.1 V versus NHE • Note that H2O reduction is kinetically slow and does not interfere • Gold • Pt • Gold SAMs hexanethiol on gold Kolb and coworkers, Langmuir (2001) Potential and Current Flow: non-Faradaic Ideal Polarized Electrode Applying a potential causes charge rearrangement: excess charge on electrode surface and ion charge near electrode (electrode double layer) Negative potential Potential of Zero Charge - + - +- + +- - ++ - + - + Positive potential +- + +- ++ -+ + + - -+ + + - ++ + - +- + + + - C = Q/E and Q = σM x area Potential and Current Flow: non-Faradaic Ideal Polarized Electrode Applying a potential causes charge rearrangement: excess charge on electrode surface and ion charge near electrode (electrode double layer) Q=CE i = dQ/dt i = C (dE/dt) Q = σM * area No direct charge transfer across capacitor, but current flows whenever the potential changes. Potential and Current Flow: non-Faradaic Electrode Double Layer Typically it is divided into an inner layer (also called compact, Helmholtz, Stern) and an outer layer (also called diffuse layer, ….) IHP OHP σS = σi + σd = -σM + – + + – + + - σi σd Define IHP and OHP as centers of charge. Diffuse layer is > OHP and Stern layer is < OHP. Double Layer Potential Profile Solve the Poisson-Boltzmann Eqn: 𝑑2𝜙 𝑑𝑥 2 =− 𝑒 𝜀𝜀0 𝑖 𝑛𝑖 𝑧𝑖 𝑒𝑥𝑝 − 𝑧𝑖 𝑒𝜙 𝑘𝑇 and solve for the potential via 𝑡𝑎𝑛ℎ 𝑧𝑒𝜙/4𝑘𝑇 = 𝑒𝑥𝑝 −𝜅 𝑥 − 𝑥2 𝑡𝑎𝑛ℎ 𝑧𝑒𝜙2 /4𝑘𝑇 so that the capacitance is 1 𝑥 = 2 + 𝐶𝑑 𝜀𝜀0 1 𝜅 𝜀𝜀0 𝑐𝑜𝑠ℎ 𝑧𝑒𝜙2 /2𝑘𝑇 Potential and Current Flow: non-Faradaic Model the electrochemical cell by a combination of circuit elements. Potential and Current Flow: non-Faradaic Imagine a potential step experiment We begin with the system at equilibrium and E=0, then we ‘rapidly’ jump the potential to E. Q = Cd x EC and E = ER + EC so that E = i Rs + Q/Cd or dQ −Q E = + dt Rs Cd Rs which gives the result i = E/Rs ∙ exp(-t/(RsCd)) and q = Ecd [1- exp(-t/RsCd))] Potential and Current Flow: non-Faradaic Imagine a potential step experiment We begin with the system at equilibrium and E=0, then we ‘rapidly’ jump the potential to E. i = E/Rs * exp(-t/(RsCd)) and q = Ecd [1- exp(-t/RsCd))] Potential and Current Flow: non-Faradaic Imagine a potential sweep experiment Let us vary the potential in a triangle waveform and measure the current. Potential and Current Flow: Faradaic Origin of Faradaic Current Changes in the charge state of atoms and molecules Potential and Current Flow: Faradaic Ideal Polarizable Electrode versus Ideal Nonpolarizable electrode Potential and Current Flow: Faradaic Factors affecting Faradaic Current (rxn rate) Potential and Current Flow: Faradaic Nernst Diffusion Layer When the electrode reaction is fast compared to the diffusion of species to the surface, a depletion layer is formed. The two cases (1 and 2) correspond to two potentials Potential and Current Flow: Faradaic Steady-State Voltammogram for Nernstian Reaction The oxidant being present initially . in the For thecase caseof ofonly both the reductant and oxidant present initially solution, one finds that E = E1/2 +(RT/nF) ln((il-i)/i) and the limiting current is il = n F A (DO/d0) C*O At the half-wave potential (il = il/2), then E = E1/2 =E0’ - (RT/nF) ln(mO / mR ) Potential and Current Flow: Faradaic Cyclic Voltammograms and Kinetics We will discuss this topic next time. A Case Study with Steady-State Photocurrent & a Slow Rxn Goal: Determine the distance dependence of the electron tunneling. Method: A) Prepare monolayer films of alkanethiols. B) Measure the photocurrent for different alkane chain lengths. InP Electrochemical Characterization 5 C8 4 3 A j = kHT CD ps 2 C12 Pt 1 C16 0 -0.8 -0.7 -0.6 -0.5 -0.4 -0.3 -0.2 -0.1 0.0 0.1 0.2 Volts (vs SCE) - Mott-Schottky analysis gives flatband of -0.7 V (vs. SCE) - Photocurrent onset is -0.65 V (vs. SCE) 0.3 0.4 InP SAM redox couple Concentration Dependence of Photocurrent Bare Electrode Fe(CN)63-/Fe(CN)64- in 0.5 M K2SO4 8 photocurrent (mA) 7 6 6 5 5 4 4 3 3 2 2 1 1 0 0.003 0.008 0.013 0 0.0 0.1 0.2 0.3 Concentration (M) 0.4 0.5 Intensity Dependence of Photocurrent Bias Voltage 0.0 V vs SCE bare C10 (×50) C16 (×250) 0.5 M Fe(CN)63-/Fe(CN)64- 30 bare C10 (x50) 20 photocurrent photocurrent / nA 40 10 C16 (x250) 0 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 Intensity (mW) 1.6 1.8 2.0 Chain Length Dependence of Current Density InP/SAM/Fe(CN)63-/4- Contact 5 A 4 ln (j/A) 3 Pt 2 InP 1 = - 0.54 0 8 10 12 14 Number of Methylene 16 SAM redox couple Thickness and Tilt Angle of Chains on InP Photoelectron e-- I d ln cos Ic d InP : escape depth of photoelectron through alkanethiol, 26.7 Å for In 3d5/2 peak. Measured film thicknesses for InP/SAMs Tilt () C8 d (Å) 6.4 0.7 C12 11.1 0.6 53 3 C16 14.9 1.2 51 4 Avg = 55 ± 6 62 4 Tilt Angle and Correlate System (per CH2) ln(It/I0) Tilt angle / Hg 1.14 ± 0.09 [1] -13.68 1.08 16 2 Au(111) 1.02 ± 0.20 [2] -12.24 2.40 32 2 Au(111) 0.90 ± 0.30 [3] -11.70 3.60 27 6 -5.88 0.84 55 6 InP(100) 0.54 ± 0.07 1. Slowinski, K.; Chamberlain, R. V.; Miller, C. J.; Majda, M, JACS 1997, 119, 11910. 2. Xu, J.; Li, H-L.; Zhang, Y.; JPC 1993, 97, 11497. 3. Miller, C.; Cuendet, P.; Grätzel, M.; J.PC 1991, 95, 877. Hg studies are particularly important because tilt angle can be systematically changed. Slowinski used model with single interchain tunneling ‘hop’ allowed and found tb = 0.91 per A ; ts = 1.31 per A Tunneling Current versus Tilt Angle 0 2 interchain hops -2 βts In (I t/I 0) -4 InP/SCnCH3 -6 βtb -8 1 interchain hop -10 Au/SCnOH Au/SCnCH3 -12 -14 0 interchain hop Hg /CnCH3 -16 -18 0 20 40 Tilt angle / ° 60 80 Yamamoto etal. JPC B 2002, 106, 7469 Summary Electrochemical Cells – Definitions etc. Equilibrium properties of Echem cells – potentiometry etc. Some features of kinetic and transient measurements (more to come ….) Citations Many of the figures used in the talk are taken from two textbooks. Electrochemical Methods by Bard and Faulkner Principles of Physical Chemistry by Kuhn, Waldeck, and Foersterling Homework Assignment 1. Find an example of a potentiometric measurement and explain how the electrochemical cell operates. 2. Show that the charging current that results froma sweep in the potential of an ideally polarizable electrode at a rate of v, is given by 𝑖 = 𝑣 𝐶𝑑 1 − 𝑒𝑥𝑝 −𝑡/𝑅𝑠𝐶𝑑 3. Consider the data given in the table for the alkali ions. Write out a thermodynamic cycle and extract the Gibbs solvation energy for each of the ions. Examine the relationship between the solvation energy and the ionic radius, and compare it to the predictions of the Born model of solvation. Note that the sublimation energies are given in kJ/mol.