draytonsdatabook - MrHoffmansScienceFair
advertisement

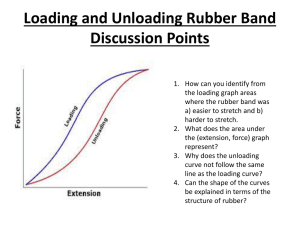
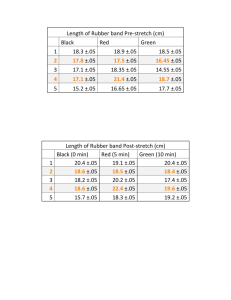
How does temperature affect the point of maximal deformation of a rubber band? Drayton Whiteside 5-6 B-Day Mr. Hoffman Table of Contents Introduction ……………………………………………………………… Page 3 Background Research …………………………………………………… Page 3 Experimental Procedures ………………………………………………... Page 3-4 Data and Results ………………………………………………………… Page 5 Discussion ………………………………………………………………. Page 6 Conclusion ………………………………………………………………. Page 6 Acknowledgments………………………………………………………… Page 7 Bibliography ……………………………………………………………... Page 7 2 Introduction I am going to find how the point of maximal deformation changes depending on the temperature. This is interesting to me because I have noticed that sometimes that when it is hot outside rubber bands expand more. So I am conducting an experiment based on my findings. I predict that heat will affect the rubber bands elasticity the most. My science fair topic falls under the branch of physics. Background Research Some background research you might want to know is that there are two types of rubber: natural rubber and synthetic rubber. By applying Hooke’s law, I realized that if I stretch a rubber band out of its elastic limit, it could change its shape permanently. Most importantly, elasticity is the quality of being elastic or being able to stretch outside of its original shape. Experimental Procedures Variables: Independent Variable: Temperature Dependent Variable: Point of Maximal Deformation Control Variables: Size, shape, thickness, and same kind of rubber bands. Time rubber bands are kept in heater/cooler, time before experimenting after taking the rubber bands out of heater/cooler, same temperature in heater, same temperature in cooler, device used for heating/cooling, and how fast or slow I stretch the rubber band to find the results. Location of Experiment: Home Materials: 18 rubber bands Measuring Tape Pencil Paper Oven Freezer Thin Cardboard Platform (paper plate cut to fit) Gloves 3 Safety Glasses Multiple Timers Aluminum Foil Platform Measurement Pointer Flat, Hard Surface Forked Metal Retaining Devices Camera Thermometers Scissors Safety Precautions: I will wear gloves that can protect me from the heat of the heater (most likely a stove). Safety glasses. Procedures: 1. Picked out 18 rubber bands that were ready for experimenting. 2. Put on safety equipment. 3. With the rubber band resting with little or no tension, measure the length (Repeat 3 times each in oven, freezer and room temperature). 4. Record the resting length of the rubber bands. 5. Put 1 rubber band on a thin cardboard platform and put in freezer at a constant temperature 32°F. 6. Keep rubber band in there for 15 minutes. 7. Take it out and stretch it over a measuring tape using the forked metal retaining devices. 8. Repeat 2 additional times. 9. Record the lengths where the rubber band snapped or went beyond its elastic limit. 10. Repeat for oven and room temperatures. 11. Record all lengths and find the averages for the different temperatures. 12. Take pictures of the experiment setup and the experiment itself. 13. Analyze data and draw conclusions. 14. Include a diagram of your experimental set-up (this can be a digital photo of your actual experiment) DON”T INCLUDE PHOTOS OF PEOPLE OTHER THAN YOURSELF>. Photographs/Pictures: Take photos of you experimental set up, data collection etc. DO NOT INCLUDE PEOPLE IN THE PHOTO UNLESS IT IS YOURSELF 4 Data and Results The data table below shows he distance the rubber band stretched with out going beyond its limit. Point of Maximal Deformation Temperature Freezer (32° F) Room Temperature (68° F) Oven (250° F) Resting Length (inches) 2⅞ 2⅞ 3.0 3⅛ 3⅛ 3⅛ 3⅛ 3.0 3⅛ PMD* Trial 1 (inches) PMD* Trial 2 (inches) PMD* Trial 3 (inches) 26 ⅜ 26 ⅜ 25 ⅞ 26 ¼ 27 ⅞ 28 ⅛ 28 ⅜ 28 ⅛ 27 ⅝ 28 ⅛ 28 ½ 28 ⅛ Average (inches) *PMD = Point of Maximal Deformation Results: The results show that room temperature and the oven temperature had the most affect to the rubber band breaking point. The freezer however, was the least affective due to the cold air shrinking the rubber band. 5 rature 6 Discussion My data did not support my initial hypothesis. The oven temperature had no affect on the point of maximal deformation, while the freezing temperature did show a change. Cold temperatures cause molecules to bunch closer together. That is why the resting length was slightly shorter under the freezing conditions. That change in the molecules of the rubber band structure directly affected the elasticity and point of maximal point of deformation, and therefore caused the rubber band to fail at a different length compared to the control. The variable that impacted my results the most was likely the speed of which I stretched the rubber band. Additionally, we used 3 trials for each data set, but if we wanted an even more accurate answer we could have had done 20 or more trials. Conclusion The final results ended with the temperatures in the oven and room temperature being the most affective toward the breaking point of rubber bands. Such information could be useful if you are constructing a rubber bungee cord for bungee jumping. If you don’t account for heat making a difference in the distance traveled by the rubber then someone could hit the ground and die from bungee jumping. Acknowledgements Thanks to my parents for buying me supplies and thanks to Mr. Hoffman and any other students who helped me out during the process. Bibliography Annotated Bibliography #1 Bibliography: Colvin, H. (2010, October 6). Rubber. Retrieved from World Book Student Database. Hartman, M. (n.d.). Polymer. Retrieved October 6, 2010, from World Book Student Database. 7 Osler, M. (2010, October 6). Robert Hooke. Retrieved from World Book Student Database. Saffman, M. (2010, October 6). Atom. Retrieved from World Book Student database. Usselman, M. (2010, October 6). Molecule. Retrieved from World Book Student Database. Summary: Rubber bands are made out of two different types of rubber. There is a natural rubber and synthetic rubber. Synthetic rubber does not perform in heat as well as natural rubber. Atoms and molecules are basic units of matter and are the building blocks of everything around us. Polymer is a large molecule formed by the chemical linking of many other smaller molecules into a long chain. Also Hooke’s law states that the amount an elastic body bends or stretches out of shape is in direct proportion to the force acting upon it. Increased stress beyond the rubber band’s limit will permanently change the shape of the elastic material. Reflection: Hooke’s law relates to my project because it tells you how the elastic consists of materials that can permanently change the shape of the rubber band. Atoms and molecules are the building blocks of matter, so my rubber bands are going to consist of atoms and molecules. I have to make sure and get both natural rubber or both synthetic rubber for my project, otherwise there will be too many variables and I will not know what affected the outcome of my results. Annotated Bibliography #2 Website- http://matse1.matse.illinois.edu/polymers/glos.html Dekker, S. A. C. (1993, May 16). Glossary. Summary: The following key terms are used in polymer production. Composite polymer is a filled or reinforced plastic, which will change the size or thickness of the rubber band. Elastomer is a type of polymer, which varies in sizes and shapes. Creep means if the polymer is cold the polymers in the rubber band will expand the rubber band. Thermo plastic is the exact opposite; it expands and softens or flows better with heat. Also Tm is a symbol I can use to symbolize melting temperature. Finally, if vulcanization was involved in producing the polymer it will toughen the polymer. Reflection: 8 These materials are going to affect my project since my problem statement is how will temperature affect the restoring force of a rubber band. Elastomer, composite polymer, and creep will come in handy for the control variables. Also thermo-plastic will affect how temperature affects the ending result, same with vulcanization. These materials will help make control variables and help to not make too many variables. Annotated Bibliography #3 Book-Temperature Sullivan, N. (2007). Temperature. Tarrytown, New York: Library of Congress Catologing-in-Publication Data. Summary: Heat is a form of energy that causes atoms and molecules to move faster than normal. Cold molecules do the opposite, they move slower than normal. There are three ways in which heat can be transferred onto another object. One of the ways is conduction where the heat energy is directly passed from one thing to another. Another way is Convection. Like Conduction, convection also has to do with the movement of material that has been heated. This form is not directly passed, for example if there was cold water in a pot on a hot stove, the stove would heat up the pot, and then the pot would heat up the water. Insulation is to keep an area a specific temperature for a set period of time. Finally Radiation is when the molecules are not touching. For example the rays from the sun come down to Earth, is a good representation of radiation. Reflection: The three different types of heat have their own effects of heating. If I use conduction I could risk flattening the rubber band and that would add another variable. If I use convection that would probably work the best because I would not be directly touching it but I would still heat it up to the same temperature every time. Also if I were to use radiation I could risk some of the heat escaping the rubber band after I finished heating it up. Lastly insulation can keep the material the same temperature for a set period of time which will allow me to not have another variable in play such as what temperature the rubber band will start at. Annotated Bibliography #4 Source: Website- Calvert, J. (2000, June 24). Elasticity. Retrieved from http://mysite.du.edu/ ~jcalvert/tech/elastic.htm Summary: Elasticity is the quality of being elastic or to be able to stretch beyond its original shape. It is also the tendency of a body of a material to return to its original form or its dimensions after being stretched. If you stretch the material beyond its elastic limit, then the material could snap or break depending on the type of substance you are dealing with. 9 Reflection: Elasticity is an important term because knowing how much something stretches, twists or bends is vital in designing machines or other structures. It is also going to help me understand why the rubber band snaps instead of stretching on and on forever. Without an elastic limit you could stretch anything for however long you wished. Finally, knowing about elasticity makes me realize that when I am doing my science fair experiment I need to stretch my rubber band at a constant rate, because if I don't then it could change the outcome of my results. Annotated Bibliography #5 Source: Chesick, J. (2010, November). Melting point. Retrieved from World Book Student database. Summary: Melting point is when a substance changes from a solid to a liquid. The melting point of a substance varies depending partly whether or not the material is a pure substance or a mixture. A pure substance melts at a specific temperature, while a mixture melts at a range of different temperatures. For example, steel melts at a range between 4000 degrees and 5000 degrees. Some pure solids do not melt when heated. Instead they change from a solid to a gas, which is called sublimation. Reflection: This information is helpful to me, because if I put a rubber band in the stove for too long, then it could start to melt. So knowing that a substance can melt at some point helps me realize that I need to make sure and have adult supervision while conducting this experiment. Otherwise I could endanger myself by getting burned or burning anything else in my house. Annotated Bibliography #6 Source: Chesick, J. (2010, November). Freezing point. Retrieved from World Book Student database. Summary: The freezing point is when the substance changes from a liquid to a solid. The freezing point can vary greatly if it's a mixture, while a pure substance's freezing point consistently stays at a specific temperature. The increase or decrease of pressure added to the material can shift the freezing point. Also, the freezing point is identical to the melting point. For example, water freezes at 0 degrees Celsius and its solid form, ice, melts at the same temperature. Reflection: 10 Freezing point is a good thing to know, because if I keep the rubber bands in a cooler temperature for too long, the rubber band might become hard and may not have its elasticity anymore. If it does not have its elasticity then it could severely change the outcome or results. 11