Effect of Water Chemistry on Jellyfish Abundance (2011)
advertisement

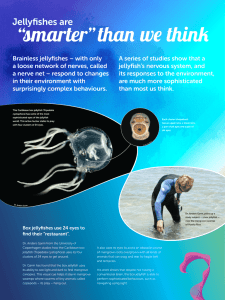
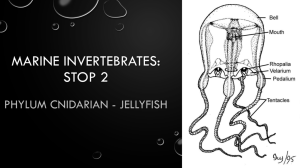
Ala Wai Research Project The Effect of Water Oxygenation, pH, Temperature and Salinity on the Numbers of Jellyfish Danielle Cournoyer Kara Onouye Maureen Barrientos Kira Wong Abstract: For the past semester, we have been testing and observing chemical properties of the water in the Ala Wai Estuary and the effect that these properties have on the jellyfish population. To understand the chemical makeup of the water, a YSI data recorder was used to measure properties such as pH, temperature, salinity, and dissolved oxygen. After data was gathered, we found fluctuations in salt concentration in the Ala Wai. The rainfall resulted in a lower, or more dilute, salinity during the winter season. The temperature, dissolved oxygen and pH all seem to vary with the seasons, and these seasonal changes seem to have an effect on the jellyfish since they appear during the times when the water is warmer, with a lower percent of dissolved oxygen and a more basic pH, or in other words during the summer. Introduction: In our experiment we aimed to study the changes in chemical properties of the Ala Wai Estuary over time. The man-made estuary is located in Waikiki and subject to sewage runoff. Jellyfish, who make the Ala Wai their home at times, are affected by the conditions of the water. Over the duration of our experiment, we compared the water conditions with the presence of jellyfish. To collect data from the water, we used a YSI. A YSI is an apparatus used to measure chemical properties of water samples (About YSI, 2010-2011). We analyzed the levels of dissolved oxygen, pH, temperature, and salinity. The data was taken at the specific depth of 1 foot throughout the 5 areas of the Ala Wai Estuary, with zone five being closest to the ocean and zone one being the furthest. Dissolved oxygen in water measures the total amount of oxygen in the water based on the diffusion of oxygen into the water from the atmosphere and as a byproduct of photosynthesis, 6CO2 + 6H2O + Energy → C6H12O6 + 6O2, in plants living in and around the water (Photosynthesis). Amount of oxygen levels in water is important for jellyfish because jellyfish are aerobic organisms, meaning they consume oxygen for metabolism processes to generate ATP, which drives cell processes within their body. The following equation demonstrates the reactants and products of cellular respiration: C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy [as ATP] (Respiration System 2010). As displayed, a low level of oxygen would result in an inability to perform respiration, and the jellyfish cannot survive in these environment. Plants and animals in the Ala Wai estuary coexist and depend on one another for the gas exchange. Plants consume carbon dioxide, combined with water and sunlight, to produce sugar and oxygen through photosynthesis. Plants absorb the waste gas of animals and provide more oxygen for cellular respiration. This is how a balance between gas exchange is maintained. PH of water is a measure of the acidity of a liquid, pH = -log10(H+). pH varies on the balance of OH- and H+ ions in the solution. An acidic environment is a result of an excess of H+ ions and is usually read as a pH level of 7 or lower. A basic environment consists of more OHions that H+ and is marked by a pH from 7 to 14. pH of water is increased based on CO2 being added to water as a byproduct of respiration from animals. Salt makes the water more basic (Acidity 2004). Considering jellyfish are ocean creatures and thrive in salt water. High pH meaning basic (not acidic) is the favorable environment for jellyfish. Salinity is the "total amount of solid materials in grams dissolved in one kilogram of sea water when all the carbonate has been converted to oxide, the bromine and iodine replaced by chlorine and all organic matter completely oxidized." However, to measure by this scientific definition is difficult. Thus, salinity is measured based on the amount of chlorine in water considering they are directly proportional; S = 0.03 + 1.805Cl. Salinity and pH are affected by evaporation of freshwater, leaving the remaining water more salty and acidic, and by rain, which raises the freshwater vs saltwater ratio and makes the water less salty and acidic. The Ala Wai specifically is connected to the ocean, which is salty, thus as the current of the ocean flows into the estuary, the water’s salinity and pH increase. Additionally, salinity can be reduced when animals use the salt in the water. Such animals include shell animals. When the shell animals take the salts out of the water, jellyfish don’t thrive as well because low levels of salt lead to flatter bodies (Sperling). Considering jellyfish receive oxygen through diffusion across the membrane of their body, flatter bodies decrease surface area and oxygen use is lowered (Lo, 2008). The last factor analyzed was temperature. Since we collected data over an extended period of time, the season changed during the course of the experiment. In this way, we were able to observe the change in temperature of the environment. The scientific definition of temperature is “a measure of the average translational kinetic energy associated with the disordered microscopic motion of atoms and molecules” (Chapter 6 2007). Jellyfish are ectotherms, meaning that they do not regulate their own body temperature, so the temperature has a great effect on them. Enzymes work quickest when temperatures rise. Additionally, mating season for jellyfish is during the summer, when temperatures are higher. Thus, we expect to see more jellyfish during the hotter temperature months (Sperling). Materials: YSI Boat pen and paper Procedure: We used the YSI meter to test the average water salinity, pH, temperature, and dissolved oxygen values. We fastened the YSI meter to a metal pole which was held in the water in front of the boat one meter below the surface of the water, and logged the values over a period of a few minutes. We recorded the times on a notepad that we spent in each zone of the estuary, and found which values correspond to which zones. These values were then averaged to find the general trends in these values between areas of the estuary. Data: Discussion: Although we planned to study the effect of various water chemistry factors on the appearance of jellyfish, we only saw jellyfish on the last day, and so can only make a hypothesis about why this is so. We decided to look at temperature, dissolved oxygen, pH and salinity of the water, since we thought that these factors would affect the numbers of jellyfish the most. At each site, we placed the YSI probe a short distance into the water, and we also used data from other groups who said that they placed the YSI at a shallow depth of one foot. The pH in each zone seemed to first decrease, then increase over the approximately seven months that we have been taking data. However, the pH in all the zones stayed between 8.2 and 6.8. Site 5 had the highest pH values, or the most basic water, while site 1 had the lowest pH values. Site 4 had slightly lower pH than Site 5, and Site 3 was also slightly lower than site 4; in other words, in the zones closer to the ocean, with a higher number, also had higher pH. This makes sense since the zones closer to the ocean likely have a higher concentration of various solutes such as salts. These salts may contribute to the pH, making the water more basic. The pH in each zone also decreases during the winter months and increases during the summer months. This is likely because it rains more during the wintertime, leading to a lower concentration of solutes in the water. Since we are taking measurements from the surface of the water, our measurements are easily affected by the weather. The estuary would be flooded with rainwater during the winter, and this rainwater could also be slightly acidic, leading to a overall more acidic pH reading in the wintertime. The rain also causes runoff, which can cause acidic trash left on the sides of the Ala Wai and substances to run into the water. The jellyfish were spotted during the summer and again recently, so the jellyfish could like the higher pH values during the summertime more than the more acidic water during the winter. The higher acidity caused by the runoff and sewage likely is not beneficial for the jellyfish, since their enzymes are optimized for the lower pH of the ocean. The temperature did not show a clear trend, although this was likely due to varying weather conditions. The temperature of the water was higher during the summer, and lower in the winter months, although it was higher in early February. Perhaps that day was uncharacteristically warm. The temperatures seem to be increasing again now that summer is nearer. The jellyfish could also prefer the warmer temperatures, explaining why we didn’t spot many jellyfish earlier in the year. The temperature could have an effect on the survival of immature jellyfish, or on the success of jellyfish mating. Jellyfish are ectotherms, meaning that they do not regulate their own body temperature, so the temperature would have a greater effect on them. Perhaps jellyfish reproduce in the summer, leading to greater numbers of jellyfish overall, and a higher probability that they would be washes into the Ala Wai, since they move with the current. The higher temperature likely allows for a higher metabolism for the jellyfish, since the higher energy allows enzymes to work quicker, and the jellyfish to grow faster. The salinity didn’t demonstrate the expected trend. We expected the salinity of the water closer to the ocean, in sites 4 and 5, to be higher than the salinity of the water farther upstream, such as in sites 1 and 2, but this was not consistently true. This was likely because of the weather, since higher amounts of precipitation could lead to a layer of fresh water on the surface of the water, where we were taking readings, which would make the salinity seem lower. Also, rain in the mountains would lead to a greater flow of water from streams into the canal, and with this greater flow of freshwater downstream there would be less diffusion of salt water upstream from the ocean. As would be expected, the salinity was lower in the wintertime, when there was more rain, and higher in the summer. The jellyfish seem to appear when the salinity is higher, which makes sense since the jellyfish normally live in the ocean where the salinity is much higher than fresher water areas of the Ala Wai. Since they are cnidarians, they are likely isotonic with the ocean, so if they go in water that is too hypotonic, they may intake too much water. The percent of dissolved oxygen was lowest in the wintertime and highest in the summertime, although warmer water usually holds less oxygen (Chapter 6, 2007). This is likely because of the rain, which would cause more water movement. Also, algae and other photosynthesizing organisms in the water would have higher photosynthesizing rates in the summer, when it is sunnier. The higher rate of photosynthesis would cause higher rates of oxygen in the water. The percent of dissolved oxygen in the water is also usually higher in zones closer to the ocean, such as four and five, than areas farther from the ocean, such as one and two. This is probably because less oxygen can be dissolved in water that already has a great deal of salt and other solutes dissolved in it (Dissolved Oxygen). The jellyfish appeared when the levels of dissolved oxygen was the highest, so the jellyfish probably make use of this oxygen for higher rates of cellular respiration. There were many possible sources of error, such as varying weather conditions and the different times of day at which the data was taken. The time of day could affect whether it was low or high tide, which would change the depth of the water and the amount of salt water entering the estuary from the ocean. We also did not record the tide level when we took the measurements, which would lead to further error. Lastly, we used data from several different groups, so slight differences in procedure, such as the depth that the YSI probe is placed at, could lead to different measurements since the salinity, temperature, pH and oxygenation values likely vary with depth. On our last work day (4/2/11), we were fortunate enough to see numerous moon jellyfish throughout the different canal zones. We tallied the jellyfish in the different zones and ended up with 1 in zone one, 13 in zone two, 25 in zone three, 6 in zone four and 137 in zone five for a total of 182 jellyfish spotted. However, a possible source of error could be that we accidentally recounted the same jellyfish more than once since there was no way to put the counted ones ‘on the side.’ Because jellyfish move slowly and are hard to see in the water it’s possible that we missed counting some of the jellyfish too. It was very interesting that out of all the work days, in both first and second semester, the jellyfish only appeared on this one day. The majority of the jellyfish spotted were seen in zone 5 at the connection point to the main canal area. This supported our hypothesis that jellyfish prefer more basic environments. However all of the jellyfish were making their way upstream however towards zone 1. As we peered into the water, we noticed that a few of the jellyfish looked like they were mating. Thus, it’s possible that the jellyfish appeared on this one day during the summertime in order to complete their mating process. Conclusion: Because jellyfish maintain their shape by hydrostatic pressure, they prefer environments that are isotonic to their bodies so they wont lose water or expand/lyse by the osmosis of water. Rainfall changes the composition of the Ala Wai water, resulting in a more dilute salinity. The pH was higher in zones 4 & 5 due to increased solute concentrations which made the water more basic. Whereas the pH was lower in the zones (1, 2, & 3) further up the canal and farther from the ocean creating a slightly more acidic environment. Though the temperature data did not show a clear trend, it was typically hotter in the summer months and cooler in the winter months. Salinity also did not demonstrate expected trends because the zones closer to the ocean did not have a higher salinity concentration compared to the zones furthest from the ocean. Fluctuations could have been caused by water runoff from the mountains lowering the salinity of the water. The amount of dissolved oxygen in the water was higher during the summer and lower during the winter. It was probably higher in the summer due to increased rates of plant photosynthesis and colder in the winter due to increased water movement from the rain. Also, the zones closer to the ocean had a lower amount of dissolved oxygen present because the water already had a large amount of dissolved solutes and salt in it. On our last work day we saw jellyfish for the first time in our data. The majority of the jellyfish were spotted in zone 5 most likely due to the fact that the water in zone 5 is more basic. We could expand these experiments by testing over the summer to see if the jellyfish do appear more when the weather is warmer and when the pH is higher. We could also observe other animals such as fish to see if salinity, temperature, amount of dissolved oxygen and pH affects them similarly and if they are more frequently found under certain conditions. Works Cited: “About YSI.” YSI. YSI Incorporated, 2010-2011. Web. 10 May 2011.<http://www.ysi.com/ about-ysi.php>. "Acidity." Ecosystem Restoration. Montana State University Bozeman , 14 Sept. 2004. 13 May 2011. <http://ecorestoration.montana.edu/mineland/guide/analytical/c hemical/water/acidity.htm>. Web. “Chapter 6: Temperature, salinity, and density.” Oceanworld. Texas A&M University, 19 July 2007. Web. 10 May 2011. < http://oceanworld.tamu.edu/resources/ocng_textbook/chapter06/chapter06_01 .htm >. "Dissolved Oxygen and Water Quality ." Commonwealth of Kentucky. N.p., n.d. Web. 10 May 2011. <http://www.state.ky.us/nrepc/water/wcpdo.htm>. Lo, Wen-Tseng. "Enhancement of jellyfish (Aurelia aurita) populations by." Oxford Journals. 2008 Web. 14 May 2011. <icesjms.oxfordjournals.org/content/65/3/453.full.pdf>. "Moon Jellyfish." Jellyfish Facts. Web. 8 May 2011. <http://www.jellyfishfacts.net/moonjellyfish.html>. "Photosynthesis." suny.edu. State University of New York, n.d. Web. 16 May 2011. <faculty.clintoncc.suny.edu/faculty/michael.gregory/files/bio%20101/bio%20101%20le ctures/photosynthesis/photosyn.htm>. "Respiratory System." Estrella Mountain Community College. 18 May 2010. Web. 10 May 2011. <http://www2.estrellamountain.edu/faculty/farabee/biobk/biobookrespsys.html>. Sperling, Kandra. "What Environment Do Most Jellyfish Live in? | eHow.com." eHow Videos, Articles & More . Web. 12 May 2011. <http://www.ehow.com/facts_5150198_environment-do-jellyfish-live.html>.