File
advertisement

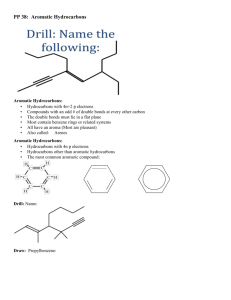
Aromatic Hydrocarbons Nomenclature of the Derivatives of Benzene Physical Properties of Aromatic Hydrocarbons Preparation of Benzene Reactions of Benzene Alkylbenzenes Nomenclature of the Derivatives of Benzene Nomenclature of Benzene Derivatives 1. Monosubstituted benzenes (a) For certain compounds, benzene is the parent name and the substituent is simply indicated by a prefix Nomenclature of the Derivatives of Benzene (b) For other compounds, the substituent and the benzene ring taken together may form a new parent name Nomenclature of Benzene Derivatives • There are three different ways that two groups can be attached to a benzene ring, so a prefix—ortho, meta, or para—can be used to designate the relative position of the two substituents. ortho-dibromobenzene or o-dibromobenzene or 1,2-dibromobenzene meta-dibromobenzene or m-dibromobenzene or 1,3-dibromobenzene para-dibromobenzene or p-dibromobenzene or 1,4-dibromobenzene 4 Nomenclature of Benzene Derivatives • If the two groups on the benzene ring are different, alphabetize the names of the substituents preceding the word benzene. • If one substituent is part of a common root, name the molecule as a derivative of that monosubstituted benzene. 5 Nomenclature of Benzene Derivatives For three or more substituents on a benzene ring: 1. Number to give the lowest possible numbers around the ring. 2. Alphabetize the substituent names. 3. When substituents are part of common roots, name the molecule as a derivative of that monosubstituted benzene. The substituent that comprises the common root is located at C1. 6 Nomenclature of the Derivatives of Benzene Nomenclature of the Derivatives of Benzene • When a substituent is one that when taken together with the benzene ring gives a new parent name, that substituent is assumed to be in position 1 and the new parent name is used Nomenclature of Benzene Derivatives • A benzene substituent is called a phenyl group, and it can be abbreviated in a structure as “Ph-”. • Therefore, benzene can be represented as PhH, and phenol would be PhOH. 9 Nomenclature of the Derivatives of Benzene Example 31-1 Draw the structural formula for each of the following compounds: (a) 1,3,5-Trichlorobenzene (b) 2,5-Dibromophenol Answer (c) 2,4-Dinitrobenzoic acid Solution: (a) (c) (b) 31.2 Nomenclature of the Derivatives of Benzene (SB p.150) Check Point 31-1 Give the IUPAC name for each of the following compounds: (a) (b) (c) (d) (a) 1,2-Dimethylbenzene (b) 1-Methyl-2-nitrobenzene or 2-nitrotoluene (c) 3-Bromo-5-chlorobenzoic acid (d) 4-Bromo-2,6-dinitrophenol Answer Give the IUPAC name for each compound. PhCH(CH3)2 isopropylbenzene OH m-butylphenol Br 2-bromo-5-chlorotoluene Cl 12 Which structure matches the given name? o-dichlorobenzene Cl Cl Cl Cl Cl Cl Br Cl A B C D Cl Cl C 13 4-chloro-1,2-diethylbenzene Cl Cl Cl Cl A B C D Cl A 14 Physical Properties of Aromatic Hydrocarbons Physical properties of aromatic hydrocarbons: • have a fragrant smell • generally less dense than water at 20°C • usually immiscible with water • soluble in organic solvents Physical Properties of Aromatic Hydrocarbons Name Formula Boiling point (°C) Melting Density at point (°C) 20°C (g cm–3) Benzene 80.1 5.5 0.878 Methylbenzene 111 –95 0.867 Ethylbenzene 136 –94 0.867 Physical Properties of Aromatic Hydrocarbons Name Formula Boiling Melting Density at point (°C) point (°C) 20°C (g cm–3) 1,2Dimethylbenzene 144 –25.2 0.880 1,3Dimethylbenzene 139 –47.4 0.864 1,4Dimethylbenzene 138 13.3 0.861 Preparation of Benzene Industrial Preparation Catalytic Reforming of Alkanes Catalytic reforming converts alkanes and cycloalkanes into aromatic hydrocarbons e.g. Pt C6H14 C6H6 + 4H2 500°C, 10 – 20 atm 31.5 Preparation of Benzene (SB p.157) Destructive Distillation of Coal • Heating coal in the absence of air gives out coal gas, ammoniacal liquor, coal tar ( )قطران الفحمand coke ()فحم الكوك • Coal tar is a mixture of many organic compounds, mainly aromatic ones • Benzene and methylbenzene can be obtained Preparation of Benzene Laboratory Synthesis Decarboxylation of Sodium Salt of Benzoic Acid When sodium benzoate is fused with sodium hydroxide, the carboxylate group is removed and benzene is formed Preparation of Benzene Reduction of Phenol Phenol vapour is passed slowly over heated zinc dust to produce benzene and zinc(II) oxide Reactions of Benzene Comparative Investigation of Chemical Properties of Cyclohexane, Cyclohexene and Benzene Reaction Cyclohexane (a saturated alicyclic hydrocarbon) Cyclohexene (an unsaturated alicyclic hydrocarbon) Methylbenzene (an aromatic hydrocarbon) Action of Br2 in CH3Cl3 (in dark) No reaction Br2 decolourized and no HBr evolved No reaction with Br2 alone In the presence of FeBr3, Br2 decolourized and HBr fumes evolved Action of H2 (with Ni catalyst) No reaction 1 mole of cyclohexene reacts with 1 mole of H2 at room temperature 1 mole of methylbenzene reacts 3 moles of H2 at high temperature and pressure Reactions of Benzene Reaction Action of acidified KMnO4 Cyclohexane (a saturated alicyclic hydrocarbon) No reaction Action of conc. No reaction HNO3 and conc. H2SO4 Cyclohexene (an unsaturated alicyclic hydrocarbon) KMnO4 decolourized Methylbenzene (an aromatic hydrocarbon) No reaction Cyclohexene A yellow liquid is oxidized and colour formed darkens Reactions of Benzene • Methylbenzene is highly unsaturated, but it is resistant to oxidation and addition reactions • The resistance of oxidation and addition reactions of aromatic compounds is used to distinguish from unsaturated alkenes • Methylbenzene reacts with Br2 in the presence of FeBr3. It is through substitution reaction Reactions of Benzene Electrophilic Aromatic Substitution Reactions Most characteristic reaction of aromatic compounds: Electrophilic substitution reactions • The electrophiles attack the benzene ring, replacing one of the hydrogen atoms in the reaction • Electrophiles are either a positive ion (E+) or some other electron-deficient species with a partial positive charge (+) Reactions of Benzene Nitration • Conc. H2SO4 increases the rate of reaction by increasing the concentration of the electrophile, NO2+ (nitronium ion) Reactions of Benzene Sulphonation • Benzene reacts with fuming sulphuric(VI) acid at room temperature to give benzenesulphonic acid • Heating aqueous solution of benzenesulphonic acid above 100°C, benzene and sulphuric(VI) acid are formed Reactions of Benzene Halogenation Benzene reacts with chlorine and bromine in the presence of catalysts such as AlCl3, FeCl3, FeBr3, to give chlorobenzene and bromobenzene respectively Reactions of Benzene Alkylation • When benzene is warmed with a haloalkane in the presence of catalysts such as AlCl3, an alkylbenzene is formed • Important step in chemical industry to produce polystyrene, phenol and detergents Reactions of Benzene Example 31-2 Complete each of the following by supplying the missing reactant or product as indicated by the question mark: Solution: (a) (a) (b) (b) conc. H2SO4, conc. HNO3 (c) (c) fuming H2SO4 Answer Reactions of Benzene Check Point 31-2 (a) One mole of benzene reacts with three moles of chlorine under special conditions. What is the reaction condition required for the reaction? (b) Draw the structure of the reaction product in (a). Answer (a) UV radiation or diffuse sunlight must be present for the free radical addition reaction to take place. (b) Alkylbenzenes • Alkylbenzenes are a group of aromatic hydrocarbons in which an alkyl group is bonded directly to a benzene ring • also known as arenes e.g. Alkylbenzenes Alkylbenzenes are oxidized to benzoic acid by strong oxidizing agents such as hot alkaline potassium manganate(VII) This type of oxidation is limited to those molecules with Alkylbenzenes Examples: Alkylbenzenes The C = C double bond and acyl groups in the side chain are oxidized by hot alkaline potassium manganate(VII) e.g. Alkylbenzenes Example 31-3 State the conditions under which methylbenzene can be converted in the laboratory to (a) C6H5CH2Cl (b) C6H5COOH Solution: (a) Reagent: Cl2 Condition: in the presence of light (b) Reagent: (1) KMnO4–, OH–, (2) H3O+ Condition: heating under reflux Answer Alkylbenzenes Check Point 31-3 Methylbenzene undergoes two different types of chlorination reaction by different mechanisms. Compare the two different types of chlorination reaction in terms of reaction conditions as well as the products formed. Answer 31.7 Alkylbenzenes (SB p.164) Two different types of chlorination reaction of methylbenzene are: Type I: free radical substitution reaction Type II: electrophilic aromatic substitution reaction Orientation effects of substituents in electrophilic aromatic substitution reactions of monosubstituted Benzenes Alkyl groups and groups with lone pairs (electron donating groups) direct new groups to ortho-, para-positions and speed-up the reaction (i.e. o & p directors and activating groups). Halogens direct new groups to ortho-, para- positions but they slow down the reaction (i.e. halogens are o & p directors and deactivating groups). Electron withdrawing groups such as nitro, nitrile, and carbonyl direct new groups to the meta-position and slow the reaction down (i.e. i.e. m directors and deactivating groups). Thus the order of reactivity of benzene and monosubstituted benzene derivatives in E.Ar.sub. is as in the following chart Substituted benzene with o,p directors > Benzene > Halobenzene derivatives > Substituted benzene with m- directors Orientation effects of substituents in electrophilic aromatic substitution reactions of monosubstituted Benzenes Ortho , para directors Meta directors -OH, -OR -NH2, -NHR, -NR2 -C6H5 -CH3, -R (alkyl) -F, -Cl, -Br, -I -NO2 -SO3H -COOH, -COOR -CHO, -COR -CN OH OH OH NO2 HNO3 / H2SO4 + o-Nitrophenol 53 % NO2 NO2 NO2 p-Nitrophenol 47 % SO3 / H2SO4 SO3H m-Nitrobezenesulfonic acid Q1: What are the major products of the following reaction? Q2: What is the final product of the following reaction? a) o-chlorobenzaldehyde b) m-chlorobenzaldehyde c) p-chlorobenzaldehyde Q3:Which one of the following compounds has aromatic character? d) a,c The END