Document
advertisement

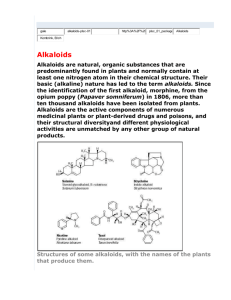
Lecture №23 Alkaloids as medicines. Sources of obtaining, methods of the structure determination. Their chemical classification, general methods of qualitative and quantitative determination. Alkaloids, imidazole’s, pyrolysidine’s, quinolysi(di)ne’s, quinoline’s derivatives, with exocyclic nitrogen atom. ass. Medvid I.I. Definition of alkaloids: Alkaloids – nitrogen containing organic bases, usually of plant origin, which have an active biological action. Alkaloids are similar to alkali. Alkaloids - complex derivatives of ammonia, which have replaced hydrogen atom on radicals: tertiary or secondary amines, or derivatives of four substituted ammonium bases. Alkaloids are weak bases. Codeine has the strongest basic properties (К= 9·10-7), caffeine – the weakest (К = 4,1·10-14). Distribution in nature: Alkaloids, related by structure, often can be found in plants that are close in botanical terms. Mainly in one plant is a mixture of alkaloids (exception – castor plant containing ricynine). Some plants (cinchona, poppy seeds, barberry) containing up to 10-15% of alkaloids. Localization - most aerial parts of medicinal plants (flowers, fruits, leaves, cortex). Some alkaloids can be moved from one part of plant to another part. Their content in plants depends on the: climate, temperature, altitude above the sea level and others. So the content of ephedrine in Ephedra may change during the year from 0.3% to 2,5%. Historic moments of alkaloid chemistry research: In 1804 the French pharmacist Sehen allocated morphine from opium as a technical product. In 1816 professor of the Kharkiv University F.I. Giza allocated quinine. In 1818 year were discovered strychnine and brucine, and a year later - caffeine. y. А.А. Voskresenskiy opened theobromine, and in 1847 J.F. – Fritzsche hormyn. In 1842 A.M. Butlerov and A.N. Vishegradskiy on the basis of their experimental work concluded that all alkaloids are derivatives of pyridine and quinoline. In 1881 y. in Russia first synthesis of coniine was conducted. 1915 y. – Chychybabin with Rodionov began industrial production of opium and other alkaloids. In 1917 y. first alkaloid plant began work in Russia. Important role in the development of chemistry played A.P. Orekhov and his school. They investigated 1500 species of plants, found more than 250 alkaloid containing plants, issued monograph "Chemistry of alkaloids”. N.А. Preobrazenskiy in 1933 at first made the original synthesis of pilocarpine. Classification of alkaloids Alkaloids are naturally occurring chemical compounds containing basic nitrogen atoms. The name derives from the word alkaline and was used to describe any nitrogen-containing base. Alkaloids are produced by a large variety of organisms, including bacteria, fungi, plants, and animals and are part of the group of natural products (also called secondary metabolites). Many alkaloids can be purified from crude extracts by acid-base extraction. Many alkaloids are toxic to other organisms. They often have pharmacological effects and use as medications and recreational drugs. Examples are the local anesthetic and stimulant cocaine, the stimulant caffeine, nicotine, the analgesic morphine, or the antimalarial drug quinine. Some alkaloids have a bitter taste. Alkaloids are usually classified by their common molecular precursors, based on the metabolic pathway used to construct the molecule. When not much was known about the biosynthesis of alkaloids, they were grouped under the names of known compounds, even some non-nitrogenous ones (since those molecules' structures appear in the finished product; the opium alkaloids are sometimes called "phenanthrenes", for example), or by the plants or animals they were isolated from. When more is learned about a certain alkaloid, the grouping is changed to reflect the new knowledge, usually taking the name of a biologically-important amine that stands out in the synthesis process. Types of the alkaloid classifications • By the chemical structure: 1) 2) 3) 4) 5) 6) 7) 8) 9) 10) derivatives of pyrrolidine (sthrahidrine, turicine) derivatives of tropane (atropine, cocaine) derivatives of pyperidine (lobeline, coniine) derivatives of pyridine (nicotine, anabasine) derivatives of pyrrolysidine (platyphylline) derivatives of quinolysidine (pahicarpine, lupinine) derivatives of quinoline (quinine) derivatives of isoquinoline (papaverine, morphine) derivatives of indol (reserpine, strychnine) derivatives of purine (caffeine, theobromine, theophylline) 11) derivatives of the different heterocycles (imidazol (pilocarpine), thiazol (agroheline), quinazoline (luotoline А), acridine (rutacridone), azenine (galantamine)); 12) polypeptide alkaloids (13-, 14-, і 15-member) (buckthorn alkaloids); 13) alkaloids with exocyclic nitrogen atom (ephedrine, muscarine, spherophysine); 14) terpenoid alkaloids (acronicyne, actinidine); 15) steroid alkaloids (solasodine, cholophyllamine). • By the ways of their biosynthesis (according to the substances from which they are obtained): a) true alkaloids (1-12 group) – which is synthezed from aminoacids and heterocycles are the base of their structure; b) protoalkaloids (13 group) – do not include heterocycles, also are the plant amines and formed from aminoacids; c) pseudoalkaloids (14, 15 group) – obtained by others ways different from aminoacids. • By plant sources Classification of alkaloids by Orekhov • Acyclic and alcaloids with exocyclic nitrogen atom (ephedrine hydrochloride, sphaerophysine benzoate, colchamine, colchicine) • Derivatives of pyrrolidine and pyrrolysidine (platyphylline hydrotartrate): N N H • Derivatives of pyridine (nicotine) and piperidine (lobeline, coniine): N N H • Condensed pyrrolidine with piperidine (tropane): N CH 3 • Derivatives of quinolysine (cytisine) and quinolysidine (pachycarpine hydroiodide): N N • Derivatives of quinoline (salts of quinine) and isoquinoline (papaverine hydrochloride, opium alkaloids): N N • Derivatives of indol (physostigmine salicilat, strychnine nitrate, reserpine): N • H Derivatives of quinazoline: N N • Derivatives of imidazol (pilocarpine hydrochloride): N N H • Derivatives of purine (caffeine, theophylline, theobromine): N N H N N • • Diterpene alkaloids (aconite, isoprenoide). Steroid alkaloids and glycoalkaloids: Nowadays, it is known more than 5000 different alkaloids, while for 3000 of them installed molecular structure. Methods of extraction from plant materials extraction in the form of salts (water, alcohol, tartaric acid); extraction in the form of basis (NH4OH, NaHCO3); Distillation of alkaloids bases with aqueous steam (boiling point for which is less than 100 º C). Extraction as salts: to raw material add water or ethanol with few drops of tartaric acid. All alkaloids forms salts with tartaric acid. For purification to this extract add base and all alkaloids form bases, which obtained by organic solutions. Operation of purification repeat few times. Then solvent separated from alkaloids. Sum of alkaloids is separated on individual compounds. Extraction as bases: to raw material add alkali solution (ammonium, sodium hydrocarbonate or carbonate). Alkaloids bases are extracted by organic solutions. Purification realize by transferring alkaloids to salts and then to bases. Operation of purification repeat few times. The methods of separation of the selected amount of alkaloids: Fractional distillation in vacuum; By the different solubility of alkaloids – salts and bases; By the different power of basic properties of alkaloids; based on the features of chemical properties; By the different ability to adsorption (chromatography); Method of anticurrent separation. For identification of alkaloids use general, group and specific reaction. The general reactions conduct with common alkaloid precipitation and special reagents. General precipitate reactions based on the ability of alkaloids as bases to give simple or complex salt with different, more often complex acids, salts of heavy metals and others. These products are usually not soluble in water, so called precipitate. General alkaloid precipitate reagents: Dragendorph reagent – BiI3+KI↔K[BiI4] SPU Lyugol, Vagner, Bushard reagents– sol. І2 in КІ in different concentraions Maier reagent – HgI2 + 2KI↔K2[HgI4] Marme reagent (solution CdI2 в KI) Zonnenshten reagent - Н3Р04 • 12Мо03 • 2Н20 Sheibler reagent - Н3Р04 • 12WоО3 • 2Н20 Berthran reagent - SiO2 • 12Wо03 • 4Н20 5% Tannin solution (freshly prepared). Saturated solution of picric acid. Special (painted) reagents on alkaloids: Conc. H2SO4. Conc. HNO3. Erdman reagent (H2SO4 conc.+HNO3 conc.). Phrede reagent ((NH4)2MoO4+H2SO4 conc.). Marki reagent (HCOH+H2SO4 conc.). Mandelin reagent (NH4VO3+H2SO4 conc.). Sodium nitroprusside (Na2[Fe(CN5)No]·2H2O). Vazitsky reagent (solution of pdimethylaminobenzaldehyde in conc. H2SO4). Methods of the quantitative determination of alkaloids: Acid-base titration in nonaqueous environment – for the quantitative determination of both salts and bases. Acid-base titration: а) acid-base titration, direct titration of acids and bases; b) acid-base back-titration for determination of bases by reverse titration; c) Alkalimetry – titration of alkaloids salts by alkali in water-alcohol medium in the presence of phenolphthalein (with or without the usage of organic solvent that does not move with water for extraction of alkaloid bases) d) Alkalimetry by the substituent Gravimetric method Methods based on individual chemical properties of alkaloids. Physico-chemical methods. Alkaloids with exocyclic nitrogen atom Ephedrine hydrochloride (Ephedrini hydrochloridum) H C H C CH3 HCl OH NHCH3 (-) 1-Phenyl-2-methylaminopropanol-1 hydrochloride Sphaerophysine benzoate (Sphaerophysini benzoas) H3C NH CH H3C C H C H N H C H2 C H2 C H2 C H2 N C 2 C6H5COOH H NH2 1-guanidino-4-(isoamylene-11)-aminobutane dibenzoate Ephedrine contains in different types of Ephedra, together with its stereoisomers L-ephedrine (cysisomer, left-rotation) C6H5 H H C C CH3 OH HN CH3 D-pseudoephedrine (trans-isomer, rightrotation) OH H C6H5 C C CH3 H HN CH3 Ephedra monosperma Obtaining of ephedrine by synthetic method Benzene with chloroanhydride of chloropropanoic acid is condensed at the presence of AlCl3 (Fridel-Crafts reaction). Obtained chloroethylphenylketone condensed with methylamine, aminoketone is formed which is reduced to ephedrine: O H C H 3C C Cl Cl C6H6 C6H5 C6H5 -HCl (H) C H C O NHCH3 CH3 C6H5 C H C O Cl H C H C CH3NH2 CH3 CH3 OH NHCH3 -HCl Sphaerophysa salsula Physical properties Ephedrine hydrochloride Colorless needle crystals or white crystalline powder, odorless, with bitter taste. Easily soluble in water (so under the action of alkali precipitate is not falls - the difference from other alkaloids), soluble in alcohol, practically insoluble in ether. Sphaerophysine benzoate White crystalline powder with bitter taste. Soluble in water, alcohol, alkalis, insoluble in ether and chloroform. Identification Ephedrine hydrochloride Sphaerophysine benzoate 1. Reactions on chlorides 2. With CuSO4 at the presence of 1. With HCl – white precipitate of benzoic acid NaOH – blue complex falls. compound (At the shaking of this solution with ether, ether 2. At the boiling with alkalis layer paints in red-violet urea separated and then NH3 color, water layer keeps blue 3. With sodium nitroprusside color). alkali solution, later HCl – 3. At the heating with potassium cherry-red color which ferrocyanide crystal smell of quickly disappears. benzaldehyde appears (bitter almond). 4. Specific rotation: from -33° to -36° (5 % water solution). Quantitative determination Ephedrine hydrochloride Sphaerophysine benzoate Acidimetric titration in nonaqueous medium in the 1) Acidimetric titration in presence of mercury (II) acetate nonaqueous medium in the (indicator crystal violet, presence of ice acetic acid Е=М.m). (indicator crystal violet, Е=М.m/2). 2) Alkalimetric titration alcoholchloroform medium (Е=М.m). 2) Bromatometry, direct titration (Е=М.m/2). 3) Argentometry by the linked HCl (Fajans’ method with the usage of bromothymol blue indicator) (Е=М.m). 1) Storage, application Ephedrine hydrochloride Sphaerophysine benzoate Drastic compound. In tightly closed container (TCC) which keeps from the action of light. Sympathomimetic (vasoconstrictive, bronchodilating) mean. By the action it is close to adrenaline, has specific stimulatory action on CNS. Internally by 0,0250,05g 2-3 times per day, i/m or i/v (intravenous) by 1 ml of 5% solution. Included to the content of Theophedrine tablets, Ephatine aerosol, Solutan and Broncholitine syrups. Drastic compound. In orange glass tightly closed container, in the place protected from lightв. Ganglioblockator mean, uterine muscle stimulant. Used for the treatment of hypertension and strengthening of maternity activity by 0,03g 23 times per day or i/m by 1ml of 1% solution. Colchamine and colchicine – alkaloids from different types of Colchicum, toxic compounds, use as ointments in the treatment of skin cancer • Colchamine • Colchicum autumnale Alkaloids – derivatives of imidazol Pilocarpine hydrochloride (Pilocarpini hydrochloridum) C2H5 C H2 O O N CH3 HCl N α-Ethyl-β-(1-methylimidazollyl-5-methyl)γ-butyrolactone hydrochloride (3S,4R)-3-Ethyl-4-[1-methyl-1Н-imidazol-5-yl)methyl]dihydro-3Нfuran-2-one hydrochloride 5(3-ethyl-4,5-dihydrofuranone-2)-methylene-1-methylimidazol hydrochloride Obtaining of pilocarpine Pilocarpus Jaborandi Was obtained in 1875 y. Diminished in size dry leaves extracted by acidified alcohol. Distilled alcohol from the extract and separated free alkaloids which transfer to nitrates and then to hydrochlorides. Alkaloids are separated by the factional crystallization or chromatographic method. Pilocarpine was synthezed in 1933 y. by А.M. Preobrazenskiy from homopilopic acid, which obtained from diethyl ether of ethylamber acid С2Н5СН(СООС2Н5)СН2СООС2Н5 by the following scheme: Physical properties of pilocarpine hydrochloride Optical active, has two asymmetric carbon atoms. Colorless crystals or white crystalline powder, odorless. Hygroscopic. It is easily soluble in water, easily soluble in alcohol, practically insoluble in ether and chloroform. Identification of pilocarpine hydrochloride 1. 2. 3. Substance gives reaction on chlorides. Chelch sample. Reaction of the formation of above chromic acids (mixture Н2О2, Н2SО4 conc., К2Сr2О7) and chromoperoxide (CrO5), which with pilocarpine base forms blue-violet complex compound soluble in chloroform. At the absence of pilocarpine colored product is not extracted by chloroform. Legal reaction on lactone ring. With sodium nitroprusside in alkali medium – cherry-red color, which does not disappear when you add excess of chloride acid. This reaction can be used for the photocoloeimetric determination of pilocarpine in 1 % water solutions. 4. Specific rotation from +88,5° to +91,0° (2 % water solution). 5. Hydroxame reaction (presence of the lactone ring – butyrolactone): 6. Preparation at the grinding with calomel becomes black as a result of formation of metallic mercury at the pilocarpine oxidation: Pilocarpine Pilopic acid+ Methylurea Quantitative determination of pilocarpine hydrochloride 1. Acid-base titration in nonaqueous medium in the presence of mercury (II) acetate (Е=М.m). 2. Alkalimetry in alcohol medium (Е=М.m). 3. Iodometry, reverse titration (after the separation of polyiodide precipitate). Storage Poison compound. In tightly closed container, which keeps from the light and moisture. Application Cholinolytic (myotic) mean. Prescribed as eye drops (1-2% solution) or ointment for the treatment of glaucoma. Alkaloids – derivatives of pyrrolysidine Platyphylline hydrotartrate (Platyphyllini hydrotartras) CH3 CH3 H3C C H C C C H2 O C H O C OH C O O CH2 N HO HOOC H C C H COOH OH To establish the structural formula of platyphylline you should study the products of its hydrolysis. At the heating with alcoholic solution of alkali platyphylline decomposes on aminoalcohol platynecyne and synecionilic acid: So, platyphylline – cyclical diester, in which two hydroxyl groups of platynecyne are etherificated by synecionilic acid. Senecio plathyphylus Platyphylline and his companion seneciphylline are derivatives of 1- methylpyrolysidine, was extracted in 1935 y. by О. P. Orekhov і R. А. Conovalova from the roots and herb of Senecio plathyphylus. Properties of platyphylline hydrotartrate A white odorless crystalline powder with weak or specific smell and bitter taste. Easily soluble in water, very little soluble in alcohol, practically insoluble in chloroform and ether. Identification of platyphylline hydrotartrate 1. Speciofic rotation from -38° to -40° (5 % water solution). 2. With Dragendorph reagent forms orange precipitate. 3. With Mayer reagent forms white precipitate. 4. Formation of iron (III) hydroxamate red color (ester group). 5. Substance gives reaction on tartrates: a) with potassium salts – white crystal precipitate; b) with 0,1 М AgNO3 solution – white precipitate. To the one part of solution add dil. НNO3 – precipitated dissolves; at the heating of second part of precipitate with NH4OH on the walls of the tube forms silver mirror”, cases by the properties of tartaric acid; c) with β-naphthalene in the presence of Н2SО4 conc. Green color appears at heating; if instead β-naphthalene used resorcinol – red-violet color of aurine dye-stuff : HO H OH + HO OH C H2SO4 CH + -2H2O OH OH O OH HO H2SO4 C -2H2O OH O Aurine dye-stuff àóðè í î âè é áàðâí è ê Quantitative determination of platyphylline hydrotartrate 1. Acid-base titration in nonaqueous medium, a direct titration, the indicator - crystal violet (Е=М.m). 2. Alkalimetry in alcohol-chloroform medium (Е=М.m). 3. Iodometry, back-titration (by the reaction of formation of polyiodide in saturated solution of NaCl). 4. Photocolorimetry – on the basis of the reactions with general alkaloid color reagents. 5. Extraction-photometric – determination of platyphylline hydrotartrate in the injection solution and tablets by the reaction with tropeoline 000-ІІ. 6. UV- spectrophotometry, GLCH. Purity test Seneciphylline - unacceptable impurity: there should be no distraction while adding of 5% ammonia solution. Storage Poison compound. In tightly closed container. In dry place. Application m-Cholinolytic (spasmolytic, midriatic) mean. At the spasms of smooth muscles of the abdominal cavity, spasms of blood vessels, bronchial asthma and others. Highest one-time dose. - 0,01 g, highest daily dose - 0,03 g. Subcutaneous (s/c) 0,2% 1,0 ml, eye drops -1%. Alkaloids – derivatives of quinolysine Cytisine (Cytisinum) NH N O Derivative of 1,2,3,4 tetrahydroquinolysine-6, condensed with piperidine White or slightly yellowish crystalline powder. Easily soluble in water, alcohol and chloroform. Cytisine is extracted from seeds by 60% alcohol acidified by acetate acid. Extract is evaporated to the dry state, added to the alkali residue and extract alkaloids by chloroform. Chloroform extract evaporated to the dry state and separated alkaloids by factional crystallization or usage of the ion-exchange resins. Substance is purified by the distillation in vacuum. Cytisus laburnum Thermopsis (Thermopsis lanceolata) Identification of cytisine 1. By the physico-chemical constants: melting point, specific rotation. 2. Nitration reaction of the aromatic ring with subsequent reduction of the nitro-group to the amino-group and azodye formation. 3. With solution of cobalt (II) nitrate - blue-green sediment. 4. With solution of iron (III) chloride - red color, which disappears when you add water. 5. The reaction of alkaloids with Dragendorph reagent. O2N NH HNO3 NH N [H] N O N H2N NH N+ O NaNO2 NH HCl N Cl- N O O O N HO N NaO NaOH N NH Quantitative determination of cytisine Acid-base titration in aqueous medium, a direct titration, the indicator - methyl red (Е=М.m). Nitrogen atom in piperidine cycle is titrated. Storage Poison compound. Protect from moisture. Application Stimulant of blood circulation and respiration. From cytisine produced 0,15 % water solution of cytitone for injections. Cytisine is a part of the tablets against smoking “Tabex”. Alkaloids – derivatives of quinolysidine Pachycarpine hydroiodide (Pachycarpini hydroiodidum) N CH2 N HI or N HI N d-Sparteine hydroiodide The pachycarpine structure contains two fused quinolysidine cycles White crystalline powder. Easily soluble in chloroform, soluble in alcohol and water, difficult soluble in ether and acetone. Pachycarpine is obtained from the aerial parts of Sophora pachycarpa Sophora pachycarpa Storage Drastic compound. In the protected from light place. Application As ganglioblockator mean, uterine muscle stimulant. Use for the treatment of hypertension and spasms of peripheral vessels in the dosage of 0,05-01 g (oral); for the uterine muscle stimulantion – 3-5 ml of 3 % solution (s/c, i/m). Identification of pachycarpine hydroiodide 1. Substance gives reactions on iodides. 2. Allocation of the phachicarpine base which can be identified by the following reactions : 3. а) by the formation of pachycarpine picrate (yellow precipitate, melting point ); б) by the reaction of interaction with pairs of bromine and ammonia on the filtrate paper - appears pink color after heating: R•HI + Br2 + NH3 R + NH4I + NH4Br + I2 4. With alkali solution of sodium nitroprusside - red-brown fine crystalline precipitate which is dissolved in the excess of HCl. 5. Specific rotation from +8,6° to +9,6° (7 % solution in alcohol), Quantitative determination of pachycarpine hydroiodide 1. Acid-base titration in nonaqueous medium, direct titration in the presence of mercury (II) acetate, indicator - crystal violet (Е=М.m/2). 2. Argentometry, Fajans method, indicator - sodium eosinate (Е=М.m). Alkalimetry in alcohol medium by thymolphthalein в (Е=М.m). Photocolorimetry. 3. 4. Alkaloids – derivatives of quinoline To this group alkaloids of quinine cortex belong – 24 alkaloids, the main representative of which is quinine: HC CH2 HO CH H3CO N 3 H2O N 6'-Methoxyquinoline-(4')-[5-vinylquinuclidine-(2)]-carbinol Quinine sulfate (Chinini sulfas) Quinini sulfas* HC Quinine hydrochloride (Chinini hydrochloridum) Quinini hydrochloridum* HO CH CH2 N H3CO HC CH2 * H2SO4 * 2H2O HO N CH neutral salt N H3CO * HCl *2H2O N neutral salt Quinine dihydrochloride (Chinini dihydrochloridum) HC HO CH N H3CO * 2HCl N Acidic salt Cinchona (Cinchona Remija) CH2 Obtaining Cortex crushed and mixed with a mixture of lime and NaOH (for the transferring of the alkaloids salts to the free basis), then extracted at 60-65оС by organic solvents. Extract washed by Н2SО4. From aqueous solution occurs sediment - quinine sulfate, which is purified by crystallization. Other alkaloids of cinchona cortex are divided by the help of ion-exchange resins. From quinine sulfate by exchange with salts of Ва2+ quinine salts are obtained. Properties Salts of quinine - colorless crystalline substances, odorless, have very bitter taste. Gradually becomes yellow at the action of light. All of them are left-rotation isomers. Solubility Qunine dihydrochloride - very easily soluble; quinine hydrochloride - soluble, and quinine sulfate - a little soluble in water. Identification of quinine salts 1. Distinguished reaction - on anions of the corresponding salts: chlorides or sulfates. 2. Reaction on alkaloids with Dragendorph. 3. Solutions of all quinine salts at the acidification by dil. H2SO4 give blue fluorescence in UV - light. 4. Specific rotation of 3 % solutions of salts in 0,1 М solution of hydrochloric acid by the calculation on dry basis is : quinine dihydrochloride - 225°; quinine hydrochloride - 245°; quinine sulfate - 240°. 5. At the interaction of alcohol solution of salt, acidified by H2SO4, with alcoholic solution of iodine formed characteristic (as leaves), green crystals of herepatite 4C20H24O2N2 · 2H2SO4 · 2HI · I4 · 6H2O. 6. The general reaction - thaleyoquine test: to the solution of quinine salt add a few drops of bromine water and ammonia - appears emerald green color: HC HC CH2 Br HO HO CH N O Br2 H3CO N HC CH2 OH OH HO CH N HN Thaleyoquine Òàë åé î õ³í N N NH4OH O N NH CH CH2 Br 7. Erythroquine reaction. At the action of bromine water and potassium hexacyanoferrate (ІІІ) in alkali medium on the quinine solution red color appears. This reaction is in 10 times more sensitive then thaleyoquine, but color quickly disappears. R H3CO O 5 R H3CO - Br2; OH ; K3[Fe(CN)6] 7 H N R O H N R O H3CO H3CO 7 N H N R - õ³í óê ë ³äè í î âè é ô ðàãì åí ò õ³í ³í ó O - H2 R-quinuclidine fragment of quinine R H3CO 5 Åð³òðî õ³í N Erythroquine Quantitative determination of quinine salts Gravimetric method which based on the precipitation of quinine base by the NaOH solution, its extraction by chloroform and weighing of the residue obtained after the distillation of chloroform. Percentage content by the calculation on the dry matter is calculated by the following formula: X = (mв.ф.•F•100)/mhatch. 100/(100-В) F – gravimetric factor, F = М.mquinine salt/М.mquinine base В – mass fraction of moisture , % 2. Quinine salts in the medical forms are determined by alkalimetry in neutralized by phenolphthalein mixture of alcohol and chloroform : X • HCl + NaOH X + NaCl + H2O (Е = М.m.) (X)2 • H2SО4 + 2NaOH 2X + Na2 SО4 + 2H2O (Е = М.m./2) 3. Acidimetry in nonaqueous medium. 4. Bromatometry, direct titration. (Е = М.m./2). KBrO3 + 5KBr + 6HCl → 3Br2 + 6KCl + 3H2O; HC HC CH2 Br CH2 Br HO HO CH N CH Br2 H3CO N H3CO . N N Purity test The specific impurity in quinine hydrochloride is barium – solution acidified by HCl, should not become turbid within 2 hours after the adding of dil. H2SО4. Storage In tightly closed container, which keeps from action of light. Aplication Antimalaral medicine. Stimulant of uterine muscles (quinine sulfate and quinine hydrochloride). Quinine sulfate: powder, tabl. 0,15 and 0,5 g; 1,0-1,2 g per day internally for the treatment of malaria. Quinine hydrochloride: tabl. 0,25 and 05 g; Quinine dihydrochloride: 50 % solution 1,0 ml Thank you for attention!