jssc4030-sup-0001-SupMat
advertisement

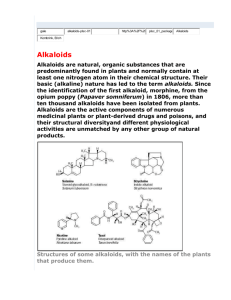
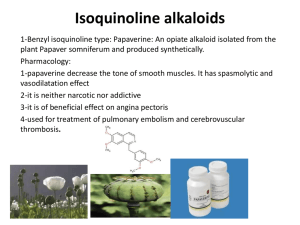
Simultaneous content determination of isoquinoline alkaloids in Dicranostigma leptopodum (Maxim) Fedde and its effective fraction of the alkaloids by high performance liquid chromatography coupled with diode array detector Yali Chena,b, Min Lia, Jianjun Liuc, Qian Yana, Mei Zhonga, Junxi Liua,*, Duolong Dia, Jinxia Liud a Key Laboratory of Chemistry of Northwestern Plant Resources and Key Laboratory for Natural Medicine of Gansu Province, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, PR China b Institute of Medicinal Chemistry, School of Pharmacy, Lanzhou University, Lanzhou 730000, PR China c University hospital of Gansu Traditional Chinese Medicine,Lanzhou 730000, PR China d Institute of Biology, Gansu Academy of Sciences, Lanzhou 730000, PR China * Corresponding author. Tel.: +86 931 4968212; Fax.: +86 931 8277088. E-mail address: liujx@licp.cas.cn (J.X. Liu) 3 Results and discussion 3.1 Selection of chromatographic conditions The quantitative determination of such alkaloids by chromatographic methods was always a challenge in analytical chemistry. This is due to the formation of hydrogen bonds between the N atoms of the alkaloid, especially the N atom of the quaternary protoberberine, and the silanol groups (R3N…H–OSi) of the separation medium, which can cause tailing of the analytes. This tailing in reversed phase (RP) chromatography is a problem that commonly occurs in the separation of alkaloids, and often affects the analytical resolution, sensitivity, and reproducibility of the method. For this reason, the quantitative determination of alkaloids requires a stationary phase that is packed with a highly pure silica gel with a low level of silanol activity (by the use of endcapped bonded phases). Not surprisingly, new-generation columns can be fully endcapped to decrease the number of surface silanols and ensure low silanol activity, which could improve their ability to separate alkaloids. Of these new-generation columns, the Waters XTerra MS C18 (250 mm × 4.6 mm, 5 μm) and the SinoChrom ODS-BP C18 (250 mm × 4.6 mm, 5 μm) columns were selected for optimization of the chromatographic conditions. The former column was more suitable for the separation of the quaternary protoberberine alkaloids, but was not suitable for all four types of isoquinoline alkaloids. It was also more suitable for separating alkaloids at lower pH values of the mobile phase so the SinoChrom ODS-BP C18 column was selected for the subsequent experiments. Alkaloids are generally basic, nucleophilic compounds containing one or two nitrogen atoms and tend to readily accept a proton and become ionized in acidic conditions. When analysis was performed at neutral pH or in moderately basic conditions, both the protonated form and the free base form of the alkaloid could co-exist, resulting in an irregular peak shape. It has already been reported that, in alkaline medium, quaternary protoberberine alkaloids could be converted into their free bases while protopine and aporphine alkaloids could be converted into their OH group adduct forms. Therefore, the pH of the mobile phase was the most important factor in the RP-HPLC separation of basic alkaloid compounds. In the present study, the maximum absorbance of the extracts and the reference compounds was observed on the UV spectrum in the range of 200–400 nm. The IS shows three maxima at 218, 270 and 305 nm (see Fig. S4-Fig. S11), and CO shows three maxima at 213, 270 and 307 nm. PR and AL, which have similar molecular structures, show three maxima at 205, 230, and 284 nm. The quaternary protoberberine alkaloids all show three UV absorptance wavelength (BE: 239, 270 and 360; HY: 237, 268 and 358 nm; BR: 229, 265 and 347 nm). Considering the relatively high contents of BE in the sample extracts and the influence of the mobile phase at other absorption bands, 270 nm was selected as the detection wavelength for all but the quaternary protoberberine alkaloids, which were detected at 360 nm. The HPLC-DAD chromatograms of DLF under the optimized chromatographic conditions are shown in Fig. S2. Figure S1. Chromatogram of standard solution. Conditions: 270 and 360 nm; Mobile phase: acetonitrile (A) and 0.2% phosphoric acid in water, adjusted by triethylamine solution to pH 6.32 (B); Gradient: 20–26% (A) at 0-20 min, 26–50% (A) at 20-35 min, 50% (A) at 35-37 min, 50–20% (A) at 37-40 min, and 20% (A) at 40–45 min; Injection volume: 20 L; Flow rate: 1.0 mL/min. Figure S2. Chromatogram of DLF extract at different detection wavelength (Chongxin, Gansu, China, May). A: 270 nm; B: 360 nm; C: Enlarged images for y-axis from 0 to 150 mAU at 270 nm. HPLC conditions are the same as for Figure S1. Figure S3. Picture of Dicranostigma leptopodum (Maxim) Fedde taken at Pingliang Kongtong mountain national geopark on May 25, 2012. Figure S4. UV spectra of BE. max at 239 nm, 270 nm and 360 nm. Figure S5. UV spectra of PR. max at 205 nm, 230 nm and 284 nm. Figure S6. UV spectra of AL. max at 205 nm, 230 nm and 284 nm. Figure S7. UV spectra of HY. max at 237 nm, 268 nm and 358 nm. Figure S8. UV spectra of SI. max at 209 nm and 243 nm. Figure S9. UV spectra of BR, max at 229 nm, 265 nm and 347nm. Figure S10. UV spectra of CO. max at 213 nm, 270 nm and 307nm. Figure S11. UV spectra of IS. max at 218 nm, 270 nm and 305 nm.