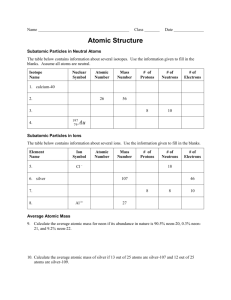
9 Reading the Periodic Table_adj2

Reading the
Periodic Table
Dmitri Mendeleev
(1869)
In 1869 Mendeleev and Lothar Meyer (Germany) published nearly identical classification schemes for elements known to date. The periodic table is base on the similarity of properties and reactivities exhibited by certain elements. Later, Henri
Moseley ( England,1887-1915) established that each elements has a unique atomic number, which is how the current periodic table is organized.
What are some similarities and differences from today ’ s periodic table vs. Mendeleev ’ s table?
Creating
A
Table
Creating a Table
Purpose: The purpose of this lesson is to acquaint you with the organization of the elements by allowing you to create your own table from the patterns you see in the elements.
Instructions
1. Work in groups of three with one set of periodic table cards.
2. Find Be, Mg, Ca, and Sr from the deck of cards, and arrange them in a column like the one on the right. Look for similar characteristics in that group.
3. With your team, decide how you can use the remainder of the cards to organize the elements into a similar pattern on the right.
4. Arrange the cards and answer the following questions on a white board.
Following Up Questions
1. What characteristics did you use for sorting the cards.
2. Did you notice any cards that didn quite fit, or seemed out of order?
Explain your thinking.
’ t
3. Where did you put H and He? What was your reasoning for their placements?
Following Up Questions
1. What characteristics did you use for sorting the cards.
• Color
• Size
• Number of “ sticks ”
• Atomic mass
• How reactive it is
• State of matter
• Metal or non-metal or metalloid
2. Did you notice any cards that didn quite fit, or seemed out of order?
Explain your thinking.
’ t
3. Where did you put H and He? What was your reasoning for their placements?
Following Up Questions
4. Why do some elements have a red , black or green ring around it?
Red =gases green = liquid black =solid
Following Up Questions
5. What patterns or characteristics do you see emerging in “ your ” periodic table?
*Diggin ’ Deeper Question*
What properties would the missing
“ element ” in row 4 have? Germanium
• Green color on the card
• Four “ sticks ”
• Reacts very slowly with oxygen
• Silver solid
• Found in GeH
4
Remember when…
In seventh grade they said all matter is made up of small “ particles ” . We call them ATOMS !
And that these “ particles ” , now called ATOMS , were made up of even smaller “ particles!
”
These smaller particles are called…
“ Subatomic Particles ” and there are three of them.
• Protons
• Electrons
• Neutrons
What does Atomic Mass tell us?
The number of Protons and Neutrons
Do you see any problems here?
How could you find the number of neutrons???
Answer:
Atomic mass (rounded )
Atomic #
# of neutrons
Welcome!
1. Please grab a worksheet to the left of the screen.
2. Get out your periodic tables
3. Complete the only “The Gold Dust Kid” story.
4. Use your periodic tables to complete the story.
Lets give it Particle
Inventory a Try!
How many protons, electrons, and neutrons does Mg have?
12 p +
12 e -
12 n 0
Lets give it Particle
Inventory a Try!
How many protons, electrons, and neutrons does Na have?
11 p +
11 e -
12 n 0
Let ’ s Practice Particle Inventory…
Find the number of p + , e , and n for each element:
Gold
1. Au
79 p +
79 e
118 n
-
Potassium
2. K
19 p
19 e -
20 n
+
Terbium
3. Tb
65 p
65 e
94 n
+
-
4. Be
5. B
6. C
7. N
8. O
9. F
Particle Inventory
Find the number of p + , e , and n for each element: p=4 e=4 n=5 p=5 e=5 n=6 p=6 e=6 n=6 p=7 e=7 n=7 p=8 e=8 n=8 p=9 e=9 n=10
Particle Inventory
Find the number of p + , e , and n for each element:
10. Cs
11. Hg
12. Cu
13. Ta
14. Br
15. Es
16. Md
17. W
18. Pb
19. Rn
Particle Inventory
Find the number of p + , e , and n for each element:
10. Cs
11. Hg
12. Cu
13. Ta
14. Br p= 55 e= 55 n= 78 p= 80 e= 80 n= 121 p= 29 e= 29 n= 35 p= 73 e= 73 n= 108 p= 35 e= 35 n= 45
15. Es
16. Md
17. W
18. Pb
19. Rn p= 99 e= 99 n= 153 p= 101 e= 101 n= 157 p= 74 e= 74 n= 110 p= 82 e= 82 n= 125 p= 86 e= 86 n= 136
Particle Inventory Warm Up
Particle Inventory Yb La V Os Eu
30. Yb p= 70 e= 70 n= 103
31. La
32. V
33. Os
34. Eu p= 57 e= 57 n= 82 p= 23 e= 23 n= 28 p= 76 e= 76 n= 114 p= 63 e= 63 n= 89
What is a reoccurring pattern when looking at the particle inventory data above?
1. Protons and Electrons numbers are always the same.
20. Sr
21. Po
22. Al
23. Si
24. P
Particle Inventory Practice
Find the number of p + , e , and n for each element: p= 38 e= 38 n= 50 p= 84 e= 84 n= 125 p= 13 e= 13 n= 14 p= 14 e= 14 n= 14 p= 15 e= 15 n= 16
25. S
26. Cl
27. Ar
28. At
29. Ca p= 16 e= 16 n= 16 p= 17 e= 17 n= 18 p= 18 e= 18 n= 22 p= 85 e= 85 n= 125 p= 20 e= 20 n= 20
Particle Inventory Practice
Particle Inventory Am Fr Cd Bi Kr
35. Am p= 95 e= 95 n= 148
36. Fr
37. Cd
38. Bi
39. Kr p= 87 e= 87 n= 136 p= 48 e= 48 n= 64 p= 83 e= 83 n= 126 p= 36 e= 36 n= 48