Cell and Molecular Biology
advertisement

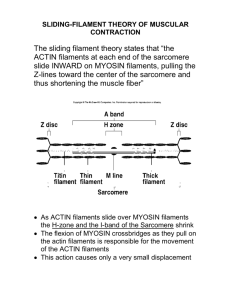
Cell and Molecular Biology Cytoskeleton-1 Behrouz Mahmoudi www.soran.edu.iq 1 The cytoskeleton Is a dynamic 3-dimensional structure that fills the cytoplasm, and is present in both eukaryotic and prokaryotic cells. The cytoskeleton acts as both muscle and skeleton, and plays a role in cell protection, cell motility (migration), cytokinesis, intracellular transport, cell division and the organization of the organelles within the cell Cytoskeleton has three main structural components: microfilaments, intermediate filaments, and microtubules. www.soran.edu.iq 2 Actin filaments (also called microfilaments) Monomers of the protein actin polymerize to form long, thin fibers that are about 8 nm in diameter. Functions: provide mechanical strength to the cell link transmembrane and cytoplasmic proteins anchor centrosomes during mitosis generate locomotion in cells interact with myosin to provide the force of muscular contraction www.soran.edu.iq 3 Protein folding www.soran.edu.iq 4 Microfilaments are solid rods made of a protein known as actin When it is first produced by the cell, actin appears in a globular form (G-actin) creating a filamentous form of the protein (F-actin) Each microfilament exhibits polarity, the two ends of the filament being distinctly different. This polarity affects the growth rate of microfilaments, one end (termed the plus end) typically assembling and disassembling faster than the other (the minus end) Actin can hydrolyze its bound ATP to ADP + Pi, releasing Pi. The actin monomer can exchange bound ADP for ATP. The conformation of actin is different, depending on whether there is ATP or ADP in the nucleotide-binding site www.soran.edu.iq 5 G-actin (globular actin) with bound ATP can polymerize, to form Factin (filamentous actin). F-actin may hydrolyze its bound ATP to ADP + Pi and release Pi. ADP release from the filament does not occur because the cleft opening is blocked. ADP/ATP exchange: G-actin can release ADP and bind ATP, which is usually present in the cytosol at higher concentration than ADP www.soran.edu.iq 6 Filament growth at one end, designated plus (+), exceeded that at the other end, designated minus (-). In electron micrographs, bound myosin heads appear as arrowheads pointing toward the negative end of the filament. Actin filaments may undergo treadmilling, in which filament length remains approximately constant, while actin monomers add at the (+) end and dissociate from the (-) end. This has been monitored using brief exposure to labeled actin monomers (pulse labeling) www.soran.edu.iq 7 Actin filament nucleation The initial step in the formation of an actin filament, in which actin monomers combine to form a new filament. Nucleation is slow relative to the subsequent addition of more monomers to extend the filament three main classes of protein have been identified that bypass the need for spontaneous nucleation and promote the initiation of new filament assembly. Nucleators: are the actin-related protein-2/3 (ARP2/3) complex, spire and formins. Branched actin filaments are observed in most organelles, and specific NPFs, such as WASP, N-WASP, WAVEs, WASH, and WHAMM, exist for each organelle. www.soran.edu.iq 8 Treadmilling of actin filaments can be altered by profilin and ADF which generally increase and decrease the size of actin filaments, respectively. Capping proteins: bind at the ends of actin filaments. Different capping proteins may either stabilize an actin filament or promote disassembly. They may have a role in determining filament length. For example: Tropomodulins cap the minus end, preventing dissociation of actin monomers. CapZ capping protein binds to the plus end, inhibiting polymerization. If actin monomers continue to dissociate from the minus end, the actin filament will shrink. www.soran.edu.iq 9 Cross-linking proteins: organize actin filaments into bundles or networks. Actin-binding domains of several of the cross-linking proteins (e.g., filamin, a-actinin, spectrin, dystrophin and fimbrin) are homologous. www.soran.edu.iq 10 Some actin-binding proteins such as a-actinin, villin and fimbrin bind actin filaments into parallel bundles. Depending on the length of a crosslinking protein, or the distance between actin-binding domains, actin filaments in parallel bundles may be held close together, or may be far enough apart to allow interaction with other proteins such as myosin Filamins: Dimerize, through antiparallel association of their C-terminal domains, to form Vshaped cross-linking proteins that have a flexible shape due to hinge regions. Filamins organize actin filaments into loose networks that give some areas of the cytosol a gel-like consistency. www.soran.edu.iq 11 FilGAP is a newly recognized filamin A (FLNa)-binding RhoGTPaseactivating protein. to control actin remodeling. www.soran.edu.iq 12 Spectrin: Is an actin-binding protein that forms an elongated tetrameric complex having an actin-binding domain at each end. With short actin filaments, spectrin forms a cytoskeletal network on the cytosolic surface of the plasma membrane of erythrocytes and some other cells. Ankyrins are a family of adaptor proteins that mediate the attachment of integral membrane proteins to the spectrin-actin based membrane cytoskeleton www.soran.edu.iq 13