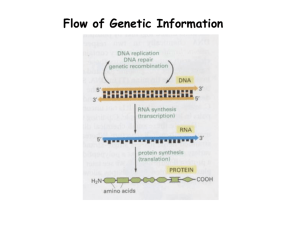
Lab Presentation
advertisement
DOES PH AFFECT THE RATE OXYGEN IS RELEASED? C By: Bekah, Emily, Miranda, & Savana Abstract • This experiment was ran to determine the optimal pH of oxygen production during the catalyzation of the enzyme catalase. • To determine the optimal pH, 3 different pH buffers were mixed with H2O2 (hydrogen peroxide), each in separate test tubes. Catalase was then added to these solutions, and a pressure gauge was plugged into the top of the test tube. As the catalase was gently agitated for 200 seconds, a graph was constructed. • To determine the optimal pH and the most oxygen produced, one must look at which pH solution had the highest pressure during catalyzation. • Our results showed that the pH solution of 9 produced the most oxygen. In conclusion, catalyzation produces the most oxygen in pH of 9. Background • Previous to this experiment, the class did an experiment that tested 4 different amounts of catalase to determine which amount of catalase was ideal for the catalyzation of 6 ml of 1.5% H2O2. The four different amounts of catalase were one, two, three, and four drops. The class found that 4 drops of catalase was ideal to catalyze 6 ml of 1.5% H2O2. This experiment led the group to find which pH level was ideal for H2O2 catalyzation. • The group chose to do an experiment on pH based group interests. The base for the experiment came from the class’ experiment. In the class experiment, the class diluted 3% H2O2 to 1.5% H2O2 by adding 3 ml of 3% H2O2 with 3 ml of distilled water. Instead of adding 3 ml of distilled water, the group added 3 ml of pH buffers with varying pH’s for the new experiment. These varying pH’s were 5, 7, and 9. The group’s goal of this experiment was to find the ideal pH of catalase to catalyze H2O2. Hypothesis • During catalyzation, the closer the buffer is to a neutral pH (7) compared to a more acidic pH (5) or a more basic pH (9), the more oxygen is produced during the first 200 seconds. • Independent variable: pH level • Dependent variable: Amount of oxygen released during the first 200 second of the experiment Method 1. First, you set up the LoggerPro program on your computer to measure the pressure of oxygen released. 2. Next you get nine test tubes for three trials and label three of them pH 5, three of them pH 7, and the last three of them pH 9. 3. Then you put three milligrams of the pH values into the correct test tubes. 4. Next you put three milligrams of 3% H2O2 (hydrogen peroxide) into all nine test tubes. 5. Now you drop four drops of catalase into the first tube for your first trial. 6. Immediately after, you place the stopper into the test tube and press collect on the LoggerPro program and gently agitated the solution for the duration of the collection. 7. After 200 seconds you take out the stopper and measure the linear fit of the line from 50-200 seconds. 8. Next you save your graph then repeat steps 5-7 for all three pH level for all three trials. Results • After completing our experiment the results were a little varied. We believe this variation can be due to human error mainly. But after looking closely we noticed that the line with the pH level of 9 rose the quickest within the first 200 seconds of the experiment. The line that increased the second fastest was the pH level of 7 and the line that rose the slowest was the acidic pH of 5. • We determined which line rose the fastest using the linear fit tool on Logger Pro. In all trials but one the line with the pH of 9 had the highest linear fit. Discussion • In two of the three runs, a pH of 9 had the highest catalyzation rate. Following the basic pH of 9 is a neutral pH of 7, then an acidic pH of 5. The group decided this true because a pH of 7 had a higher catalyzation rate in two of the three runs opposed to a pH of 5. • The findings of the group shows that when catalase is used to catalyze H2O2, the pH of the H2O2 mixture should be 9 for the highest oxygen production. • The group’s experiment did achieve the group’s goal. Although, the results proved the group’s hypothesis incorrect. The results showed that a pH of 9 was ideal for catalyzation as opposed to a pH of 7, as stated in the hypothesis. Discussion Cont. • Errors in this experiment could have been caused by human error or because of limited time. • Human errors in this experiment could have been because of differentiated agitation of each of the 9 different test tubes. This error could have been avoided there were stirrers used. • Limited time could have caused errors in this experiment because the catalase had to sit for 3 days. The group used the catalase solution the day they started the experiment for the first two runs. They then used the catalase solution 3 days later for the third run of the experiment. This could have caused some errors due to the time the catalase solution sat unused. • Further experiments may include testing temperature and pH in the same experiment to determine if temperature changes the ideal pH and testing more pH’s ranging from 1-14 to find a more specific pH rather than testing pH’s of only 5, 7, and 9. Conclusion • During catalyzation, the closer the buffer is to a basic pH level of 9, compared to a more neutral pH of 7 or a more acidic pH of 5, the more oxygen is produced within the first 200 seconds of the experiment.