Part I

Chapter 6: Free Electron Fermi Gas
Free Electron Theory
•
Conductors fall into 2 main classe s; metals & semiconductors.
Here, we focus on metals.
•
A metal is loosely defined as a solid with valence electrons that are not tightly bound to the atoms but are relatively easily able to move through the whole crystal.
• The room temperature conductivity of metals is in the range
( RT ) metals
;10
6
10
8 m and it increases on the addition of small amounts of impurities. The resistivity normally decreases monotonically with decreasing temperature & can be reduced by the addition of small amounts of impurity.
Question! Why do mobile (conducting) electrons occur in some solids & not in others?
•
When the interactions between electrons are considered,this becomes a
Very Difficult Question to Answer
.
D
Some Common Physical Properties of Metals:
Great Physical Strength, High Density, Good
Electrical and Thermal Conductivity, etc.
•
Here, we outline a surprisingly simple theory which does a very good job of explaining many of these common properties of metals.
“The Free Electron Model”
•
We begin with the assumption that conduction electrons exist & that they consist of the valence electrons from the metal atoms.
•
Thus, metallic Na, Mg & Al are assumed to have
1, 2 & 3 mobile electrons per atom, respectively.
•
This model seems at first as if it is
Way Too Simple!
•
But, this simple model works remarkably well for many metals & it will be used to help to explain many properties of metals.
• According to this
Free Electron Model (FEM)
the valence electrons are responsible for the conduction of electricity, & for this reason these electrons are called
“Conduction Electrons”.
• As an example, consider Sodium (Na).
• The electron configuration of the Free Na Atom is:
1s 2 2s 2 2p 6 3s 1
Valence Electron
Core Electrons
(loosely bound)
• The outer electron in the third atomic shell
(n = 3, ℓ = 0) is the electron which is responsible for the chemical properties of Na.
• When Na atoms are put together to form a Na metal,
Na metal
•
•
Na has a BCC structure & the distance between nearest neighbors is
3.7 A˚
• The radius of the third shell in Na i is
1.9 A˚
In the solid the electron wavefunctions of the Na atoms overlap slightly. From this observation it follows that a valence electron is no longer attached to a particular ion, but belongs to both neighboring ions at the same time.
• Therefore, these conduction electrons can be considered as moving independently in a square well of finite depth& the edges of the well correspond to the edges of the sample.
• Consider a metal with a cubic shape with edge length
L :
Ψ and E can be found by solving the Schrödinger equation:
Since
2
2 m
2
E
V
V
0
Use periodic boundary conditions
& get the
Ψ’s as travelling plane waves.
-L/2 0 L/2
( x
,
,
L )
( , , )
•
The solutions to the Schrödinger equation are plane waves,
1
V e i k r
1
V e x
y
z
)
Normalization constant
•
Na
p
Na
p
2
k
2
where V is the volume of the cube, V=L 3 k
2
Na p
2
L p
So the wave vector must satisfy k x
2
L p k y
2
L q k z
2
L r where p, q, r taking any integer values; +ve, -ve or zero.
•
The wave function Ψ(x,y,z) corresponds to an energy
E
2 2 k
2 m
E
2
2 m
( k x
2 k y
2 k z
2 )
• The corresponding momentum is: p
( , , x y z
)
• The energy is completely kinetic:
1
2 mv
2
2 2 m v
2 2 k
2 m
2 2 k p
k
• We know that the number of allowed k values inside a spherical shell of k-space of radius k is:
Vk
2
2
2 dk ,
• Here g(k) is the density of states per unit magnitude of k .
Number of Allowed States per Unit Energy Range?
• Each k state represents two possible electron states, one for spin up, the other is spin down.
dk
( )
2 ( )
( )
2 ( ) dE
E
2 2 k
2 m dE
2
dk m k k
2 mE
2
( )
2
V
2 k k 2 mE
2
2 dE k
2
V
2 3
(2 )
3/ 2 1/ 2 m E
Ground State of the Free Electron Gas
•
•
Electrons are Fermions (s = ± ½) and obey the Pauli exclusion principle ; each state can accommodate only one electron.
The lowest-energy state of N free electrons is therefore obtained by filling the N states of lowest energy.
• Thus all states are filled up to an energy E
F
, known as The Fermi energy , obtained by integrating the density of states between 0 and
E
F ,
The result should equal N.
Remember that
2
V
2 3
(2 )
3/ 2 1/ 2 m E
E
F
N
( )
0
E
F
0
2
V
2 3
(2 )
3/ 2 1/ 2 m E dE
3
V
2 3
(2 mE
F
)
3/ 2
• Solving for E
F
( Fermi energy );
E
F
2
2 m
3
V
2
N
2 / 3
• The occupied states are inside the Fermi sphere in kspace as shown below; the radius is Fermi wave number k
F
. k z
E
F
2
2 m
3
V
2
N
2 / 3
E
F
2 2 k
F
2 m e
Fermi Surface
E = E
F k
F k y
From these two equations, k
F found as, can be k
F
3
2
N
1/ 3
V k x
The surface of the Fermi sphere represents the boundary between occupied & unoccupied k states at absolute zero for the free electron gas.
• Typical values may be obtained by using monovalent potassium metal ( K ) as an example; for potassium the atomic density and hence the valence electron density
N/V is 1.402x10
28 m -3 so that
E
F
19
J
2.12
eV k
F
0.746
A
1
• The Fermi (degeneracy) Temperature T
F is given by
E
F
k T
B F
T
F
E
F
k
2.46 10 4
B
K
•
•
•
It is only at a temperature of this order that the particles in a classical gas can attain (gain)
kinetic energies as high as E
F
Only at temperatures above T
F
.
will the free electron gas behave like a classical gas.
Fermi momentum
P
F
k
F
P
F
m V e F
•
•
V
F
P
F
0.86 10 m e
6 ms
1
These are the momentum & velocity values of the electrons at the states on the Fermi surface of the Fermi sphere.
So, the Fermi Sphere plays an important role in the behavior of metals.
Typical values for monovalent potassium metal;
E
F
2
2
3
m V
2
N
2 / 3
2.12
eV k
F
3
V
2
N
1/ 3
0.746
A
1
V
F
P
F
m e
6 ms
1
T
F
E k
F
B
2.46 10
4
K
Free Electron Gas at Finite Temperature
• At a temperature T the probability of occupation of an electron state of energy E is given by the
Fermi distribution function f
FD
1
1 e
( E
E
F
) / k T
• The Fermi distribution function determines the probability of finding an electron at energy E.
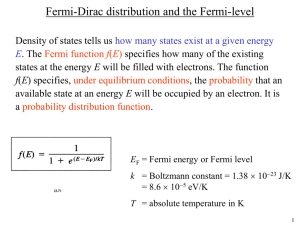
Fermi-Dirac Distribution & The Fermi-Level
Main Application: Electrons in a Conductor
•
The Density of States g(E) specifies
how many states exist at a given energy E .
•
The Fermi Function f(E) specifies how many of the
existing states at energy E will be filled with electrons.
E
F
= Fermi Energy or Fermi Level k = Boltzmann Constant
T = Absolute Temperature in K
•
I
•
The Fermi Function f(E) specifies, under
equilibrium conditions, the probability that an available state at an energy E will be occupied by an electron. It is a probability distribution function .
20
Fermi-Dirac Statistics
β
(1/kT)
The Fermi Energy E
F is essentially the same as the
Chemical Potential
μ.
E
F is called The Fermi Energy .
Note the following :
• When
E = E
F
, the exponential term = 1 and F
FD
• In the limit as
T → 0:
= (½).
L
L
• At T = 0 , Fermions occupy the lowest energy levels.
• Near
T = 0 , there is little chance that thermal agitation will kick a Fermion to an energy greater than E
F
.
Fermi-Dirac Distribution
Consider T
0 K
For E > E
F
: f ( E
E
F
)
1
1
exp (
)
For E < E
F
: f ( E
E
F
)
1
1
exp (
)
0
1
E
A step function!
E
F
0 1 f(E)
0.5
f
FD
(E,T)
Fermi Function at T=0
& at a Finite Temperature
f
FD
1
e
(
1
F
) / k T f
FD
=? At 0°K a. E < E
F f
FD
1
e
(
1
F
) / k T
1 b. E > E
F f
FD
1
e
(
1
F
) / k T
0
E
E<E
F
E
F
E>E
F
Fermi-Dirac Distribution
Temperature Dependence
Fermi-Dirac Distribution: Consider T > 0 K
If E = E
F then f(E
F
) = ½ .
If
1 kT f ( E )
exp
( E
E
F
)
So, the following approximation is valid: kT
That is, most states at energies 3kT above E
F
If then
F
3 kT exp
E kT
E
F
1 are empty.
l
So, the following approximation is valid: f ( E )
1
exp kT
E
F
So, 1
f(E) = probability that a state is empty, decays to zero.
So, most states will be filled. kT (at 300 K) = 0.025eV, E g
(Si) = 1.1eV, so 3kT is very small in comparison to other energies relelevant to the electrons.
25
Fermi-Dirac Distribution
Temperature Dependence
T = 0
T > 0
The Fermi “Temperature” is defined as
T
F
≡ (E
F
)/(k
B
).
T = 0
T > 0
As the temperature increases from T = 0,
The Fermi-Dirac Distribution
“smears out”.
T = T
F
T >> T
F
•
•
As the temperature increases from T = 0,
The Fermi-Dirac Distribution “smears out”.
When T >> T
F
, F
FD exponential.
approaches a decaying
At T = 0 the Fermi Energy E
F is the energy of the highest occupied energy level .
•
If there are a total of N electrons , then is easy to show that E
F has the form:
N
1
3
(
E
F
E
1
)
3 / 2
E
F
E
1
(
3 N
)
2 / 3 h
2
8 m
3 N
(
L
3
)
2 / 3
Fermi-Dirac Distribution Function
e
1
( )
/
1
1.2
1.0
0.8
F
/ k
B
T
T = 0
50
20
10
0.6
0.4
0.2
0.0
0.00
0.25
0.50
0.75
1.00
1.25
1.50
( k )/
F
T-Dependence of the Chemical Potential
Classical
0
-2
-4
0 1 2
Temperature
3
•
•
Number of electrons per unit energy range according to the free electron model?
The shaded area shows the change in distribution between absolute zero and a finite temperature.
n(E,T)
T=0 g(E)
• n(E,T)
number of free electrons per unit energy range is just the area under n(E,T) graph.
T>0
( , )
( )
FD
( , )
E
E
F
• The Fermi-Dirac distribution function is a symmetric function; at finite temperatures, the same number of levels below E
F by electrons.
are emptied and same number of levels above E
F are filled n(E,T) g(E)
T=0
T>0
E
E
F
k x k z
Fermi Surface
k y k
F
3
2 n
1/ 3
F
h
2 3 n 2 / 3
2 m 8
T
F
F
/ k
B
U u
3 N
3
F
5
F
5
Fermi-Dirac Distribution Function
1.4
1.2
e
1
( )
/
1 dn d
1.0
0.8
0.6
0.4
F
/ k
B
T = 50
T
T
F
F
0.2
0.0
0.00
0.25
0.50
0.75
1.00
1.25
1.50
( k )/
F
Examples of Fermi Systems
Electrons in metals (dense system)
Heat Capacity of the Free Electron Gas
• From the diagram of n(E,T) the change in the distribution of electrons can be resembled into triangles of height
(½)g(E
F so
(½)g(E
F
)k
B
) and a base of 2k
B
T
T electrons increased their energy by k
B
T.
n(E,T)
T>0 g(E)
T=0
• The difference in thermal energy from the value at
T=0°K
( )
E (0)
1
2
(
F
)( k T
B
)
2
E
E
F
• Differentiating with respect to T gives the heat capacity at constant volume,
C v
E
T
( )
F B
2 g E k T
N
2
3
E g E
F
(
F
) g E
F
)
3 N
2 E
F
3 N
2 k T
B F
C v
Heat Capacity of
Free Flectron Gas
( )
F B
2
C g E k T v
3 N
2 k T
B
2 k T
B F
3
2
Nk
B
T
T
F
Low-Temperature Heat Capacity
C
C el
C lat
C lat
T
3
Copper
C el
2
2
T
Nk
B
T
F
• Total metallic heat capacity at low temperatures
C
T
T
3
Electronic
Lattice Heat
Heat capacity
Capacity where
γ & β are constants found plotting C v
/T as a function of T 2
Transport Properties of Conduction
Electrons
•
• Fermi-Dirac distribution function describes the behaviour of electrons only at equilibrium.
If there is an applied field ( E or B ) or a temperature gradient the transport coefficient of thermal and electrical conductivities must be considered.
Transport coefficients
σ,Electrical conductivity
K,Thermal conductivity